Diverse RNA viruses of parasitic nematodes can elicit antibody responses in vertebrate hosts
- PMID: 39232205
- PMCID: PMC11445058
- DOI: 10.1038/s41564-024-01796-6
Diverse RNA viruses of parasitic nematodes can elicit antibody responses in vertebrate hosts
Abstract
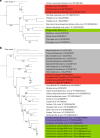
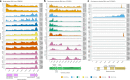
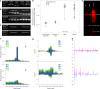
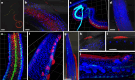
Parasitic nematodes have an intimate, chronic and lifelong exposure to vertebrate tissues. Here we mined 41 published parasitic nematode transcriptomes from vertebrate hosts and identified 91 RNA viruses across 13 virus orders from 24 families in ~70% (28 out of 41) of parasitic nematode species, which include only 5 previously reported viruses. We observe widespread distribution of virus-nematode associations across multiple continents, suggesting an ancestral acquisition event and host-virus co-evolution. Characterization of viruses of Brugia malayi (BMRV1) and Onchocerca volvulus (OVRV1) shows that these viruses are abundant in reproductive tissues of adult parasites. Importantly, the presence of BMRV1 RNA in B. malayi parasites mounts an RNA interference response against BMRV1 suggesting active viral replication. Finally, BMRV1 and OVRV1 were found to elicit antibody responses in serum samples from infected jirds and infected or exposed humans, indicating direct exposure to the immune system.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
MeSH terms
Grants and funding
- V474923N/Fonds Wetenschappelijk Onderzoek (Research Foundation Flanders)
- 11L1323N/Fonds Wetenschappelijk Onderzoek (Research Foundation Flanders)
- G0A0522N/Fonds Wetenschappelijk Onderzoek (Research Foundation Flanders)
- BB/V011278/1/RCUK | Biotechnology and Biological Sciences Research Council (BBSRC)
LinkOut - more resources
Full Text Sources

