Can we counterbalance restricted access to innovation through specialized breast cancer care? The REAL-NOTE study
- PMID: 39232267
- PMCID: PMC11403271
- DOI: 10.1016/j.breast.2024.103793
Can we counterbalance restricted access to innovation through specialized breast cancer care? The REAL-NOTE study
Abstract
Introduction: The KEYNOTE-522 (KN-522) trial showed that the addition of pembrolizumab to standard chemotherapy improved pathological complete response (pCR) and event-free survival (EFS) for patients with early triple negative breast cancer (TNBC). We analyzed results of a real-world cohort of patients treated in a certified Breast Unit, before the introduction of pembrolizumab, to see if high quality care can match outcomes brought by the addition of an innovative anticancer therapy.
Methods: Observational, retrospective, single-center cohort study, with real-world data from an ongoing institutional database with prespecified variables. Inclusion criteria matched the ones from KN-522: previously untreated stage II or III TNBC, diagnosed between 2012 and 2022, who received neoadjuvant chemotherapy. The primary endpoints were pCR at the time of definitive surgery and EFS; overall survival (OS) was a secondary endpoint.
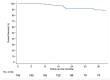
Results: Total of 168 patients were included, median age 55 years, 55 % received neoadjuvant chemotherapy with dose dense anthracyclines and taxanes and 25 % carboplatin + paclitaxel, sequenced with dose dense anthracyclines. Most had Stage II disease (82.7 %), 47 % node + disease. pCR was achieved in 52.7 % cases. At 36 months, EFS was 83.3 % (95 % CI 75.1-89.0) and OS 89 % (95 % CI, 81.6 to 93.5).
Conclusions: Notwithstanding the study limitations, outcomes of patients treated with chemotherapy without immunotherapy were numerically similar to the experimental arm of KN-522 trial. These data highlight that providing care by a specialized multidisciplinary team in a certified unit might be just as impactful as the incorporation of new technologies.
Keywords: Early Breast Cancer; Neoadjuvant treatment; Quality of care; Triple Negative Breast Cancer.
Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Merino M.J. Gattuso's differential diagnosis in surgical pathology [internet] Elsevier; 2022. Breast; pp. 721–762.https://linkinghub.elsevier.com/retrieve/pii/B9780323661652000132 Available from:
-
- Harbeck N., Penault-Llorca F., Cortes J., Gnant M., Houssami N., Poortmans P., et al. Breast cancer. Vol. 5. Nat Rev Dis Prim. 2019 Sep 23;5(1):66. - PubMed
-
- Schmid P., Cortes J., Pusztai L., McArthur H., Kümmel S., Bergh J., et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810–821. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources