Elacestrant in ESR1-mutant, endocrine-responsive metastatic breast cancer: should health authorities consider post hoc data to inform priority access?
- PMID: 39232441
- PMCID: PMC11403265
- DOI: 10.1016/j.esmoop.2024.103701
Elacestrant in ESR1-mutant, endocrine-responsive metastatic breast cancer: should health authorities consider post hoc data to inform priority access?
Abstract
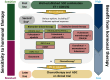
For patients with hormone receptor-positive/human epidermal growth factor receptor 2 (HER2)-negative (HR+/HER2-) metastatic breast cancer (mBC) progressed on first-line endocrine therapy plus a cyclin-dependent kinase 4 and 6 inhibitor (CDK4/6i), fulvestrant, a selective estrogen receptor degrader (SERD) administered intramuscularly, represented the only monotherapy option until the approval of elacestrant. This oral SERD has been approved for patients with ESR1-mutant HR+/HER2- mBC by the European Medicines Agency, the Food and Drug Administration, and the UK Medicines and Healthcare products Regulatory Agency, according to the results of the randomized phase III EMERALD trial, which demonstrated elacestrant superiority over standard endocrine monotherapy. Consequently, elacestrant has been incorporated in the European Society for Medical Oncology and American Society of Clinical Oncology guidelines. However, in Europe, the access to this recommended drug depends on the decision of the National Health Authorities of each state. In this communication, we describe the main results and implications of the EMERALD trial, in the context of the biomarker-driven algorithm for patients with HR+/HER2- mBC progressed on CDK4/6i, and conclude that a subgroup of patients with ESR1-mutant tumors and specific clinical features can really derive a clinically meaningful benefit from elacestrant, sparing access to more toxic combination approaches and preserving the quality of life.
Keywords: elacestrant; hormone receptor-positive/HER2-negative breast cancer; metastatic breast cancer.
Copyright © 2024 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Eurostat Cancer statistics—specific cancers. 2024. https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Cance... Available at.
-
- Loibl S., Poortmans P., Morrow M., Denkert C., Curigliano G. Breast cancer. Lancet. 2021;397(10286):1750–1769. - PubMed
-
- Gennari A., André F., Barrios C.H., et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32(12):1475–1495. - PubMed
-
- Rugo H.S., Lerebours F., Ciruelos E., et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): one cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021;22(4):489–498. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

