CMG901, a Claudin18.2-specific antibody-drug conjugate, for the treatment of solid tumors
- PMID: 39232496
- PMCID: PMC11528232
- DOI: 10.1016/j.xcrm.2024.101710
CMG901, a Claudin18.2-specific antibody-drug conjugate, for the treatment of solid tumors
Abstract
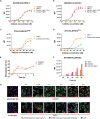
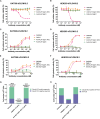
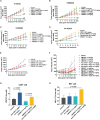
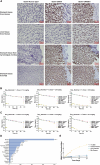
Claudin18.2 has been recently recognized as a potential therapeutic target for gastric/gastroesophageal junction or pancreatic cancer. Here, we develop a Claudin18.2-directed antibody-drug conjugate (ADC), CMG901, with a potent microtubule-targeting agent MMAE (monomethyl auristatin E) and evaluate its preclinical profiles. In vitro studies show that CMG901 binds specifically to Claudin18.2 on the cell surface and kills tumor cells through direct cytotoxicity, antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and bystander killing activity. In vivo pharmacological studies show significant antitumor activity in patient-derived xenograft (PDX) models. Toxicity studies show that the major adverse effects related to CMG901 are reversible hematopoietic changes attributed to MMAE. The highest non-severely toxic dose (HNSTD) is 6 mg/kg in cynomolgus monkeys and 10 mg/kg in rats once every 3 weeks. CMG901's favorable preclinical profile supports its entry into the human clinical study. CMG901 is currently under phase 3 investigation in patients with advanced gastric/gastroesophageal junction adenocarcinoma expressing Claudin18.2 (NCT06346392).
Keywords: ADC; CMG901; Claudin18.2; solid tumors.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests G.X., W.L., Y.W., Y.H., L.Z., Q.S., C.W., and B.C. are employees of Keymed Biosciences (Chengdu) Limited. The patents disclosed included WO2022078523A1 and its patent family.
Figures

Similar articles
-
Therapeutic efficacy, pharmacokinetic profiles, and toxicological activities of humanized antibody-drug conjugate Zt/g4-MMAE targeting RON receptor tyrosine kinase for cancer therapy.J Immunother Cancer. 2019 Mar 14;7(1):75. doi: 10.1186/s40425-019-0525-0. J Immunother Cancer. 2019. PMID: 30871619 Free PMC article.
-
DB-1310, an ADC comprised of a novel anti-HER3 antibody conjugated to a DNA topoisomerase I inhibitor, is highly effective for the treatment of HER3-positive solid tumors.J Transl Med. 2024 Apr 17;22(1):362. doi: 10.1186/s12967-024-05133-7. J Transl Med. 2024. PMID: 38632563 Free PMC article.
-
An EGFR-targeting antibody-drug conjugate LR004-VC-MMAE: potential in esophageal squamous cell carcinoma and other malignancies.Mol Oncol. 2019 Feb;13(2):246-263. doi: 10.1002/1878-0261.12400. Epub 2018 Nov 15. Mol Oncol. 2019. PMID: 30372581 Free PMC article.
-
Emerging Claudin18.2-targeting Therapy for Systemic Treatment of Gastric Cancer: Seeking Nobility Amidst Danger.Anticancer Agents Med Chem. 2025;25(4):223-231. doi: 10.2174/0118715206329892240927081033. Anticancer Agents Med Chem. 2025. PMID: 39364863 Review.
-
Progress of Clinical Studies Targeting Claudin18.2 for the Treatment of Gastric Cancer.Dig Dis Sci. 2024 Jul;69(7):2631-2647. doi: 10.1007/s10620-024-08435-4. Epub 2024 May 20. Dig Dis Sci. 2024. PMID: 38769225 Review.
Cited by
-
Navigating the future of gastric cancer treatment: a review on the impact of antibody-drug conjugates.Cell Death Discov. 2025 Apr 5;11(1):144. doi: 10.1038/s41420-025-02429-5. Cell Death Discov. 2025. PMID: 40188055 Free PMC article. Review.
-
The role of complement in tumor immune tolerance and drug resistance: a double-edged sword.Front Immunol. 2025 Jan 31;16:1529184. doi: 10.3389/fimmu.2025.1529184. eCollection 2025. Front Immunol. 2025. PMID: 39958348 Free PMC article. Review.
-
Discordance in Claudin 18.2 Expression Between Primary and Metastatic Lesions in Patients With Gastric Cancer.J Gastric Cancer. 2025 Apr;25(2):303-317. doi: 10.5230/jgc.2025.25.e2. J Gastric Cancer. 2025. PMID: 40200874 Free PMC article.
-
The antibody-drug conjugate SHR-A1904 for targeting CLDN18.2 in advanced gastric or gastroesophageal junction cancer: a phase 1 trial.Nat Med. 2025 Jul 16. doi: 10.1038/s41591-025-03781-w. Online ahead of print. Nat Med. 2025. PMID: 40670772
-
The Icarian flight of antibody-drug conjugates: target selection amidst complexity and tackling adverse impacts.Protein Cell. 2025 Jul 19;16(7):532-556. doi: 10.1093/procel/pwaf002. Protein Cell. 2025. PMID: 39813112 Free PMC article. Review.
References
-
- Sahin U., Türeci Ö., Manikhas G., Lordick F., Rusyn A., Vynnychenko I., Dudov A., Bazin I., Bondarenko I., Melichar B., et al. FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann. Oncol. 2021;32:609–619. doi: 10.1016/j.annonc.2021.02.005. - DOI - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

