Eslicarbazepine induces apoptosis and cell cycle arrest in C6 glioma cells in vitro and suppresses tumor growth in an intracranial rat model
- PMID: 39232721
- PMCID: PMC11373099
- DOI: 10.1186/s12885-024-12840-3
Eslicarbazepine induces apoptosis and cell cycle arrest in C6 glioma cells in vitro and suppresses tumor growth in an intracranial rat model
Abstract
Background: Glioblastoma multiforme (GBM) is the most malignant brain tumor, with a poor prognosis and life expectancy of 14-16 months after diagnosis. The standard treatment for GBM consists of surgery, radiotherapy, and chemotherapy with temozolomide. Most patients become resistant to treatment after some time, and the tumor recurs. Therefore, there is a need for new drugs to manage GBM. Eslicarbazepine (ESL) is a well-known antiepileptic drug belonging to the dibenzazepine group with anticancer potentials. In this study, for the first time, we evaluated the potential effects of ESL on C6 cell growth, both in vitro and in vivo, and examined its molecular effects.
Methods: To determine the effect of ESL on the c6 cell line, cell viability, proliferation, and migration were evaluated by MTT assay, colony formation, and wound healing assay. Also, apoptosis and cell cycle were examined by flow cytometry, qRT-PCR, and western blotting. In addition, an intracranial model in Wistar rats was used to investigate the effect of ESL in vivo, and the tumor size was measured using both Caliper and MRI.
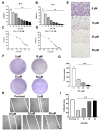
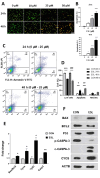
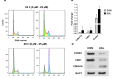
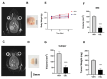
Results: The obtained results are extremely consistent and highly encouraging. C6 cell viability, proliferation, and migration were significantly suppressed in ESL-treated C6 cells (p < 0.001), as determined by cell-based assays. ESL treatment led to significant enhancement of apoptosis (p < 0.01), as determined by flow cytometry, and upregulation of genes involved in cell apoptosis, such as the Bax/Bcl2 ratio at RNA (p < 0.05) and protein levels (5.37-fold). Flow cytometric analysis of ESL-treated cells revealed G2/M phase cell cycle arrest. ESL-treated cells demonstrated 2.49-fold upregulation of p21 alongside, 0.22-fold downregulation of cyclin B1, and 0.34-fold downregulation of cyclin-dependent kinase-1 at the protein level. Administration of ESL (30 mg/kg) to male rats bearing C6 intracranial tumors also suppressed the tumor volume and weight (p < 0.01).
Conclusions: Based on these novel findings, ESL has the potential for further experimental and clinical studies in glioblastoma.
Keywords: Apoptosis; C6 cell; Caspase 3; Cytochrome c; Eslicarbazepine; G2/M cell cycle arrest; Glioblastoma; P53; p21.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Fingolimod Inhibits C6 Rat Glioma Proliferation and Migration, Induces Sub-G1 Cell Cycle Arrest, Mitochondrial and Extrinsic Apoptosis In Vitro and Reduces Tumour Growth In Vivo.Clin Exp Pharmacol Physiol. 2025 Jan;52(1):e70012. doi: 10.1111/1440-1681.70012. Clin Exp Pharmacol Physiol. 2025. PMID: 39623929
-
Flavonoids from the Amazon plant Brosimum acutifolium induce C6 glioma cell line apoptosis by disrupting mitochondrial membrane potential and reducing AKT phosphorylation.Biomed Pharmacother. 2019 May;113:108728. doi: 10.1016/j.biopha.2019.108728. Epub 2019 Mar 8. Biomed Pharmacother. 2019. PMID: 30856536
-
Anti-tumor effects of Solanum nigrum L. extraction on C6 high-grade glioma.J Ethnopharmacol. 2021 Jun 28;274:114034. doi: 10.1016/j.jep.2021.114034. Epub 2021 Mar 18. J Ethnopharmacol. 2021. PMID: 33746002
-
Naphthalimide derivative attenuates tumor growth of wild-type p53-expressing U87 glioma cells in vitro and in vivo through a biphasic dose-dependent mechanism: A switch from cell cycle to apoptosis.Biomed Pharmacother. 2025 Jun;187:118097. doi: 10.1016/j.biopha.2025.118097. Epub 2025 Apr 27. Biomed Pharmacother. 2025. PMID: 40294493
-
In vivo C6 glioma models: an update and a guide toward a more effective preclinical evaluation of potential anti-glioblastoma drugs.Rev Neurosci. 2023 Sep 1;35(2):183-195. doi: 10.1515/revneuro-2023-0067. Print 2024 Feb 26. Rev Neurosci. 2023. PMID: 37651618 Review.
Cited by
-
The potential of dibenzazepine carboxamides in cancer therapy.Front Pharmacol. 2025 Mar 28;16:1564911. doi: 10.3389/fphar.2025.1564911. eCollection 2025. Front Pharmacol. 2025. PMID: 40223925 Free PMC article. Review.
References
-
- Roura AJ, Szadkowska P, Poleszak K, Dabrowski MJ, Ellert-Miklaszewska A, Wojnicki K, Ciechomska IA, Stepniak K, Kaminska B, Wojtas B. Regulatory networks driving expression of genes critical for glioblastoma are controlled by the transcription factor c-Jun and the pre-existing epigenetic modifications. Clin Epigenetics. 2023;15(1):29. 10.1186/s13148-023-01446-4 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

