Gene therapy for polygenic or complex diseases
- PMID: 39232780
- PMCID: PMC11375922
- DOI: 10.1186/s40364-024-00618-5
Gene therapy for polygenic or complex diseases
Abstract
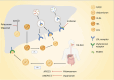
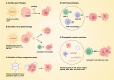
Gene therapy utilizes nucleic acid drugs to treat diseases, encompassing gene supplementation, gene replacement, gene silencing, and gene editing. It represents a distinct therapeutic approach from traditional medications and introduces novel strategies for genetic disorders. Over the past two decades, significant advancements have been made in the field of gene therapy, leading to the approval of various gene therapy drugs. Gene therapy was initially employed for treating genetic diseases and cancers, particularly monogenic conditions classified as orphan diseases due to their low prevalence rates; however, polygenic or complex diseases exhibit higher incidence rates within populations. Extensive research on the etiology of polygenic diseases has unveiled new therapeutic targets that offer fresh opportunities for their treatment. Building upon the progress achieved in gene therapy for monogenic diseases and cancers, extending its application to polygenic or complex diseases would enable targeting a broader range of patient populations. This review aims to discuss the strategies of gene therapy, methods of gene editing (mainly CRISPR-CAS9), and carriers utilized in gene therapy, and highlight the applications of gene therapy in polygenic or complex diseases focused on applications that have either entered clinical stages or are currently undergoing clinical trials.
Keywords: CRISPR-CAS9; Complex diseases; Gene therapy; Polygenic diseases.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

