Quercetin ameliorates oxidative stress-induced apoptosis of granulosa cells in dairy cow follicular cysts by activating autophagy via the SIRT1/ROS/AMPK signaling pathway
- PMID: 39232832
- PMCID: PMC11375867
- DOI: 10.1186/s40104-024-01078-5
Quercetin ameliorates oxidative stress-induced apoptosis of granulosa cells in dairy cow follicular cysts by activating autophagy via the SIRT1/ROS/AMPK signaling pathway
Abstract
Background: Follicular cysts contribute significantly to reproductive loss in high-yield dairy cows. This results from the death of follicular granulosa cells (GCs) caused by oxidative stress. Quercetin is known to have significant antioxidant and anti-apoptotic effects. However, the effect of quercetin on follicular cysts has yet been elucidated. Therefore, this study aimed to explore the anti-oxidant and anti-apoptosis effects and potential molecular mechanisms of quercetin in H2O2-induced primary cow GCs and 3-nitropropionic acid (3-NPA)-induced mouse model of oxidative stress and thus treat ovarian cysts in dairy cows.
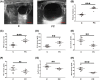
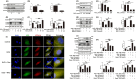
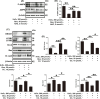
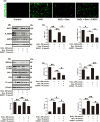
Results: In this study, compared with estrus cows, cows with follicular cysts showed heightened levels of oxidative stress and increased follicular cell apoptosis, while autophagy levels were reduced. A model of oxidative stress was induced in vitro by H2O2 and showed significant increases in apoptosis together with reduced autophagy. These effects were significantly ameliorated by quercetin. Effects similar to those of quercetin were observed after treatment of cells with the reactive oxygen species (ROS) inhibitor N-acetylcysteine (NAC). Further investigations using chloroquine (autophagy inhibitor), rapamycin (autophagy activator), selisistat (SIRT1 inhibitor), and compound C (AMPK inhibitor) showed that chloroquine counteracted the effects of quercetin on oxidative stress-induced apoptosis, while rapamycin had the same effect as quercetin. In addition, the SIRT1/AMPK pathway inhibitors antagonized quercetin-mediated mitigation of the effects of oxidative stress on increased apoptosis and reduced autophagy. Consistent with the results in vitro, in mouse ovarian oxidative stress model induced by 3-NPA, quercetin activated autophagy through the SIRT1/AMPK signaling pathway, while alleviating oxidative stress damage and inhibiting apoptosis in mouse ovaries.
Conclusions: These findings indicate that quercetin can inhibit apoptosis in GCs and restore ovarian function by activating autophagy through the SIRT1/ROS/AMPK signaling pathway, suggesting a new direction for the treatment of ovarian follicular cysts in high-yield dairy cows.
Keywords: Apoptosis; Autophagy; Follicular cyst; Oxidative stress; Quercetin.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Bijmholt S, Müller K, Leiding C, Hoedemaker M, Bollwein H, Kaske M. Lactational incidences of production diseases in German Fleckvieh cows of six Bavarian dairy farms. Tierarztliche Praxis Ausgabe G. 2012;40(6):347–58. - PubMed
LinkOut - more resources
Full Text Sources

