Updates in Alzheimer's disease: from basic research to diagnosis and therapies
- PMID: 39232848
- PMCID: PMC11373277
- DOI: 10.1186/s40035-024-00432-x
Updates in Alzheimer's disease: from basic research to diagnosis and therapies
Abstract
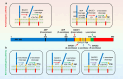
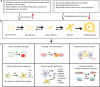
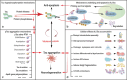
Alzheimer's disease (AD) is the most common neurodegenerative disorder, characterized pathologically by extracellular deposition of β-amyloid (Aβ) into senile plaques and intracellular accumulation of hyperphosphorylated tau (pTau) as neurofibrillary tangles. Clinically, AD patients show memory deterioration with varying cognitive dysfunctions. The exact molecular mechanisms underlying AD are still not fully understood, and there are no efficient drugs to stop or reverse the disease progression. In this review, we first provide an update on how the risk factors, including APOE variants, infections and inflammation, contribute to AD; how Aβ and tau become abnormally accumulated and how this accumulation plays a role in AD neurodegeneration. Then we summarize the commonly used experimental models, diagnostic and prediction strategies, and advances in periphery biomarkers from high-risk populations for AD. Finally, we introduce current status of development of disease-modifying drugs, including the newly officially approved Aβ vaccines, as well as novel and promising strategies to target the abnormal pTau. Together, this paper was aimed to update AD research progress from fundamental mechanisms to the clinical diagnosis and therapies.
Keywords: Alzheimer’s disease; Diagnosis; Drug development; Neurodegeneration; Tau; β-Amyloid.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- 2023 Alzheimer's disease facts and figures. Alzheimers Dement. 2023;19(4):1598-1695. - PubMed
-
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s Disease. Neurology. 1984;34(7):939–44. 10.1212/WNL.34.7.939 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

