Alpha-synuclein, autophagy-lysosomal pathway, and Lewy bodies: Mutations, propagation, aggregation, and the formation of inclusions
- PMID: 39233232
- PMCID: PMC11460475
- DOI: 10.1016/j.jbc.2024.107742
Alpha-synuclein, autophagy-lysosomal pathway, and Lewy bodies: Mutations, propagation, aggregation, and the formation of inclusions
Abstract
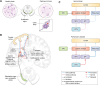
Research into the pathophysiology of Parkinson's disease (PD) is a fast-paced pursuit, with new findings about PD and other synucleinopathies being made each year. The involvement of various lysosomal proteins, such as TFEB, TMEM175, GBA, and LAMP1/2, marks the rising awareness about the importance of lysosomes in PD and other neurodegenerative disorders. This, along with recent developments regarding the involvement of microglia and the immune system in neurodegenerative diseases, has brought about a new era in neurodegeneration: the role of proinflammatory cytokines on the nervous system, and their downstream effects on mitochondria, lysosomal degradation, and autophagy. More effort is needed to understand the interplay between neuroimmunology and disease mechanisms, as many of the mechanisms remain enigmatic. α-synuclein, a key protein in PD and the main component of Lewy bodies, sits at the nexus between lysosomal degradation, autophagy, cellular stress, neuroimmunology, PD pathophysiology, and disease progression. This review revisits some fundamental knowledge about PD while capturing some of the latest trends in PD research, specifically as it relates to α-synuclein.
Keywords: Lewy bodies; Lysosomes; autophagy-lysosomal pathway; neurodegeneration; neuroimmunology; synucleinopathies; α-synuclein.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures





References
-
- Thomas B., Beal M.F. Parkinson's disease. Hum. Mol. Genet. 2007;16:R183–R194. - PubMed
-
- Smith Y., Raju D.V., Pare J.F., Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 2004;27:520–527. - PubMed
-
- Diaz-Hernandez E., Contreras-Lopez R., Sanchez-Fuentes A., Rodriguez-Sibrian L., Ramirez-Jarquin J.O., Tecuapetla F. The thalamostriatal projections contribute to the initiation and execution of a sequence of movements. Neuron. 2018;100:739–752.e735. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

