Relaxin family peptide receptor 3 (RXFP3) expressing cells in the zona incerta/lateral hypothalamus augment behavioural arousal
- PMID: 39233365
- PMCID: PMC11658188
- DOI: 10.1111/jnc.16217
Relaxin family peptide receptor 3 (RXFP3) expressing cells in the zona incerta/lateral hypothalamus augment behavioural arousal
Abstract
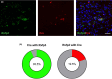
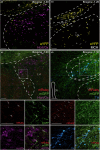
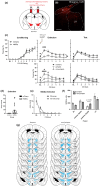
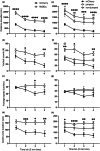
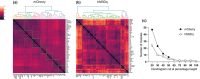
Fear-related psychopathologies, such as post-traumatic stress disorder, are linked to dysfunction in neural circuits that govern fear memory and arousal. The lateral hypothalamus (LH) and zona incerta (ZI) regulate fear, but our understanding of the precise neural circuits and cell types involved remains limited. Here, we examined the role of relaxin family peptide receptor 3 (RXFP3) expressing cells in the LH/ZI in conditioned fear expression and general arousal in male RXFP3-Cre mice. We found that LH/ZI RXFP3+ (LH/ZIRXFP3) cells projected strongly to fear learning, stress, and arousal centres, notably, the periaqueductal grey, lateral habenula, and nucleus reuniens. These cells do not express hypocretin/orexin or melanin-concentrating hormone but display putative efferent connectivity with LH hypocretin/orexin+ neurons and dopaminergic A13 cells. Following Pavlovian fear conditioning, chemogenetically activating LH/ZIRXFP3 cells reduced fear expression (freezing) overall but also induced jumping behaviour and increased locomotor activity. Therefore, the decreased freezing was more likely to reflect enhanced arousal rather than reduced fear. Indeed, stimulating these cells produced distinct patterns of coactivation between several motor, stress, and arousal regions, as measured by Fos expression. These results suggest that activating LH/ZIRXFP3 cells generates brain-wide activation patterns that augment behavioural arousal.
Keywords: RXFP3; arousal; defensive response; fear conditioning; hypothalamus; zona incerta.
© 2024 The Author(s). Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
Conflict of interest statement
Andrew J. Lawrence is the current Editor‐in‐Chief of the
Figures


References
-
- Albert‐Gascó, H. , García‐Avilés, Á. , Moustafa, S. , Sánchez‐Sarasua, S. , Gundlach, A. L. , Olucha‐Bordonau, F. E. , & Sánchez‐Pérez, A. M. (2017). Central relaxin‐3 receptor (RXFP3) activation increases ERK phosphorylation in septal cholinergic neurons and impairs spatial working memory. Brain Structure & Function, 222, 449–463. - PubMed
-
- Anversa, R. , Campbell, E. J. , Walker, L. C. , Ch'ng, S. , Muthmainah, M. , Kremer, S. , Guimarães, A. , O'Shea, M. J. , He, S. , Dayas, C. V. , Andrews, Z. B. , Lawrence, A. J. , & Brown, R. M. (2023). A paraventricular thalamus to insular cortex glutamatergic projection gates “emotional” stress‐induced binge eating in females. Neuropsychopharmacology, 48, 1931–1940. - PMC - PubMed
-
- Bankhead, P. , Loughrey, M. B. , Fernández, J. A. , Dombrowski, Y. , McArt, D. G. , Dunne, P. D. , McQuaid, S. , Gray, R. T. , Murray, L. J. , Coleman, H. G. , James, J. A. , Salto‐Tellez, M. , & Hamilton, P. W. (2017). QuPath: Open source software for digital pathology image analysis. Scientific Reports, 7, 16878. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

