Intra- and extracellular real-time analysis of perfused fibroblasts using an NMR bioreactor: A pilot study
- PMID: 39233469
- PMCID: PMC11667653
- DOI: 10.1002/jimd.12794
Intra- and extracellular real-time analysis of perfused fibroblasts using an NMR bioreactor: A pilot study
Abstract
Introduction: Metabolomic discrimination of different mitochondrial defects is challenging. We describe an NMR-based bioreactor allowing real-time intra- and extracellular metabolic investigation of perfused fibroblasts.
Objectives: The objective of this study is (I) determining whether metabolic investigations of perfused fibroblasts overall and separated for intra- and extracellular contributions by real-time NMR allows for discrimination of different representative mitochondrial defects in a feasibility study and (II) gaining insight into physiological consequences of mitochondrial dysfunction in basal condition and during glycolysis inhibition.
Methods: Overall, intra- and extracellular metabolomes of malate dehydrogenase 2 (MDH2), pyruvate dehydrogenase (PDH), complex I (CI) deficient fibroblasts, and control fibroblasts were investigated under standard culture conditions and under glycolysis inhibition. In addition to "overall" metabolite quantification, intra- and extracellular metabolic contributions were separated based on diffusion rate differences.
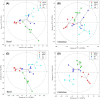
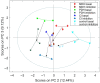
Results and discussion: Overall metabolites: Chemometric analysis of the entire metabolome revealed good separation between control, PDH and MDH2, while CI was less well separated. However, mixed intra- and extracellular changes complicated interpretation of the cellular metabolism. Intra- and extracellular metabolites: Compartment specific chemometrics revealed possibly augmenting metabolomic separation between control and deficient cell lines under basal and inhibition condition. All mitochondrial defects exhibited upregulation of glycolytic metabolism compared to controls. Inhibition of glycolysis resulted in perturbations of other metabolic pathways such as glutaminolysis, alanine, arginine, glutamate, and proline metabolism. MDH2 showed upregulation of alanine and glutamate metabolism, while the CI defect revealed lower intracellular arginine and downregulation of glutamate and arginine-dependent proline synthesis.
Conclusion: Discrimination of intra- and extracellular metabolic contributions helps understanding the underlying mechanisms of mitochondrial disorders, uncovers potential metabolic biomarkers, and unravels metabolic pathway-specific adaptations in response to metabolic perturbations.
Keywords: 2‐deoxy‐glucose; NMR; bioreactor; extracellular; intracellular; mitochondrial dysfunction.
© 2024 The Author(s). Journal of Inherited Metabolic Disease published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
Christian Urzì, Christoph Meyer, Déborah Mathis, Peter Vermathen, and Jean‐Marc Nuoffer declare that they have no conflict of interest.
Figures



References
-
- Gorman GS, Chinnery PF, DiMauro S, et al. Mitochondrial diseases. Nat Rev Dis Primers. 2016;2:16080. - PubMed
-
- Wortmann SB, Mayr JA, Nuoffer JM, Prokisch H, Sperl W. A guideline for the diagnosis of pediatric mitochondrial disease: the value of muscle and skin biopsies in the genetics era. Neuropediatrics. 2017;48:309‐314. - PubMed
-
- Meyer C, Hertig D, Arnold J, et al. Complex I, V, and MDH2 deficient human skin fibroblasts reveal distinct metabolic signatures by 1H HR‐MAS NMR. J Inherit Metab Dis. 2023;47:270‐279. - PubMed
-
- Hertig D, Felser A, Diserens G, Kurth S, Vermathen P, Nuoffer JM. Selective galactose culture condition reveals distinct metabolic signatures in pyruvate dehydrogenase and complex I deficient human skin fibroblasts. Metabolomics. 2019;15:32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

