Identification of mpox M1R and B6R monoclonal and bispecific antibodies that efficiently neutralize authentic mpox virus
- PMID: 39233480
- PMCID: PMC11413965
- DOI: 10.1080/22221751.2024.2401931
Identification of mpox M1R and B6R monoclonal and bispecific antibodies that efficiently neutralize authentic mpox virus
Abstract
In 2022, the monkeypox virus (mpox virus, MPXV) exhibited global dissemination across six continents, representing a notable challenge owing to the scarcity of targeted antiviral interventions. Passive immunotherapy, such as the use of monoclonal antibodies (mAbs) and bispecific antibodies (bsAbs), has emerged as a promising option for antiviral regimens. Here, we generated several mAbs against M1R and B6R of MPXV, and subsequently characterized the antiviral activity of these antibodies both in vitro and in vivo. Two neutralizing mAbs, M1H11 and M3B2, targeting M1R, and one B6R-specific mAb, B7C9, were identified. They exhibited varying antiviral efficacy against vaccinia virus (VACV) in vitro and in vivo. A cocktail comprising M1H11 and M3B2 demonstrated a superior protective effect in vivo. A bsAb, Bis-M1M3, was engineered by conjugating the fragment crystallizable (Fc) region of the human-mouse chimeric engineered M1H11 with the single-chain fragment variable (scFv) of M3B2. In mice challenged with MPXV, Bis-M1M3 showed a notable protective effects. Analysis of neutralization mechanism showed that these mAbs and Bis-M1M3 exerted virus-neutralizing effects before the virus infects cells. In vivo pharmacokinetic experiments showed that Bis-M1M3 has a long half-life in rhesus macaques. This study provides crucial insights for further research on broad-spectrum antiviral drugs against MPXV and other orthopoxviruses.
Keywords: Antiviral mechanism; Bispecific antibody; Monoclonal antibody; Mpox virus; Vaccinia virus.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


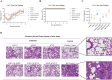
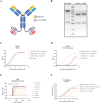


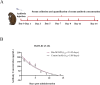
References
-
- 2022–2023 Mpox Outbreak Global Map . Available online: https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html. Accessed december 2023.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
