Dangerous liaisons: Loss of keratinocyte control over melanocytes in melanomagenesis
- PMID: 39233509
- PMCID: PMC11626500
- DOI: 10.1002/bies.202400135
Dangerous liaisons: Loss of keratinocyte control over melanocytes in melanomagenesis
Abstract
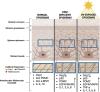
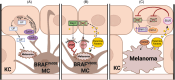
Melanomas arise from transformed melanocytes, positioned at the dermal-epidermal junction in the basal layer of the epidermis. Melanocytes are completely surrounded by keratinocyte neighbors, with which they communicate through direct contact and paracrine signaling to maintain normal growth control and homeostasis. UV radiation from sunlight reshapes this communication network to drive a protective tanning response. However, repeated rounds of sun exposure result in accumulation of mutations in melanocytes that have been considered as primary drivers of melanoma initiation and progression. It is now clear that mutations in melanocytes are not sufficient to drive tumor formation-the tumor environment plays a critical role. This review focuses on changes in melanocyte-keratinocyte communication that contribute to melanoma initiation and progression, with a particular focus on recent mechanistic insights that lay a foundation for developing new ways to intercept melanoma development.
Keywords: Melanoma; cadherin; pigmentation; tumor microenvironment; ultraviolet radiation.
© 2024 The Author(s). BioEssays published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
UV-Induced Molecular Signaling Differences in Melanoma and Non-melanoma Skin Cancer.Adv Exp Med Biol. 2017;996:27-40. doi: 10.1007/978-3-319-56017-5_3. Adv Exp Med Biol. 2017. PMID: 29124688 Review.
-
Crosstalk in Skin: Loss of Desmoglein 1 in Keratinocytes Inhibits BRAFV600E-Induced Cellular Senescence in Human Melanocytes.J Invest Dermatol. 2025 Jul;145(7):1740-1752.e4. doi: 10.1016/j.jid.2024.10.608. Epub 2024 Nov 23. J Invest Dermatol. 2025. PMID: 39581457
-
Nuclear hormone receptor functions in keratinocyte and melanocyte homeostasis, epidermal carcinogenesis and melanomagenesis.FEBS Lett. 2013 Mar 18;587(6):529-41. doi: 10.1016/j.febslet.2013.01.041. Epub 2013 Feb 5. FEBS Lett. 2013. PMID: 23395795 Free PMC article. Review.
-
Functional interplay between secreted ligands and receptors in melanoma.Semin Cell Dev Biol. 2018 Jun;78:73-84. doi: 10.1016/j.semcdb.2017.06.021. Epub 2017 Jul 1. Semin Cell Dev Biol. 2018. PMID: 28676423 Review.
-
Keratinocyte cadherin desmoglein 1 controls melanocyte behavior through paracrine signaling.Pigment Cell Melanoma Res. 2020 Mar;33(2):305-317. doi: 10.1111/pcmr.12826. Epub 2019 Oct 10. Pigment Cell Melanoma Res. 2020. PMID: 31563153 Free PMC article.
References
-
- Lee, K. J. , Soyer, H. P. , & Stark, M. S. (2024). The Skin molecular ecosystem holds the key to Nevogenesis and Melanomagenesis. Journal of Investigative Dermatology, 144(3), 456–465. - PubMed
-
- Shain, A. H. , & Bastian, B. C. (2016). From melanocytes to melanomas. Nature Reviews Cancer, 16(6), 345–358. - PubMed
-
- Harbst, K. , Lauss, M. , Cirenajwis, H. , Isaksson, K. , Rosengren, F. , Törngren, T. , Kvist, A. , Johansson, M. C. , Vallon‐Christersson, J. , Baldetorp, B. , Borg, Å. , Olsson, H. , Ingvar, C. , Carneiro, A. , & Jönsson, G. (2016). Multiregion whole‐exome sequencing uncovers the genetic evolution and mutational heterogeneity of early‐stage metastatic melanoma. Cancer Research, 76(16), 4765–4774. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

