Tumor-Homing Phage Nanofibers for Nanozyme-Enhanced Targeted Breast Cancer Therapy
- PMID: 39233557
- PMCID: PMC11733710
- DOI: 10.1002/adma.202403756
Tumor-Homing Phage Nanofibers for Nanozyme-Enhanced Targeted Breast Cancer Therapy
Abstract
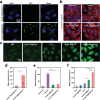
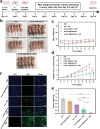
Photodynamic therapy (PDT) eliminates cancer cells by converting endogenous oxygen into reactive oxygen species (ROS). However, its efficacy is significantly hindered by hypoxia in solid tumors. Hence, to engineer filamentous fd phage, a human-friendly bacteria-specific virus is proposed, into a nanozyme-nucleating photosensitizer-loaded tumor-homing nanofiber for enhanced production of ROS in a hypoxic tumor. Specifically, Pt-binding and tumor-homing peptides are genetically displayed on the sidewall and tip of the fd phage, respectively. The Pt-binding peptides induced nucleation and orientation of Pt nanozymes (PtNEs) on the sidewall of the phage. The resultant PtNE-coated tumor-homing phage exhibits significantly enhanced sustained catalytic conversion of hydrogen peroxide in hypoxic tumors into O2 for producing ROS needed for PDT, compared to non-phage-templated PtNE. Density functional theory (DFT) calculations verify the catalytic mechanism of the phage-templated PtNE. After intravenous injection of the PtNE-coated indocyanine green (ICG)-loaded tumor-homing phages into breast tumor-bearing mice, the nanofibers home to the tumors and effectively inhibit tumor growth by the PtNE-enhanced PDT. The nanofibers can also serve as a tumor-homing imaging probe due to the fluorescence of ICG. This work demonstrates that filamentous phage, engineered to become tumor-homing nanozyme-nucleating tumor-hypoxia-relieving nanofibers, can act as cancer-targeting nanozymes with improved catalytic performance for effective targeted PDT.
Keywords: hydrogen peroxide decomposition; hypoxic tumor; phage nanofibers; photodynamic therapy; platinum nanozyme.
© 2024 The Author(s). Advanced Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- a) Wang X.‐S., Zeng J.‐Y., Zhang M.‐K., Zeng X., Zhang X.‐Z., Adv. Funct. Mater. 2018, 28, 1801783;
- b) Zhang Y., Wang F., Liu C., Wang Z., Kang L., Huang Y., Dong K., Ren J., Qu X., ACS Nano 2018, 12, 651. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

