Patients with Persistent Mild Psoriasis after Treatment with Ustekinumab Achieved Greater Improvements in Skin Clearance and Patient-reported Outcomes after Switching to Guselkumab in the Phase 3 NAVIGATE Trial
- PMID: 39233617
- PMCID: PMC11388105
- DOI: 10.2340/actadv.v104.41053
Patients with Persistent Mild Psoriasis after Treatment with Ustekinumab Achieved Greater Improvements in Skin Clearance and Patient-reported Outcomes after Switching to Guselkumab in the Phase 3 NAVIGATE Trial
Abstract
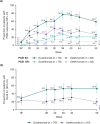
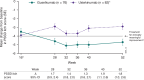
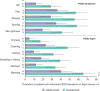
Mild psoriasis may be burdensome; if symptoms are inadequately controlled, switching therapy may be warranted. In the Phase 3 NAVIGATE trial, patients with moderate-to-severe plaque psoriasis received ustekinumab for 16 weeks. Patients with inadequate response (Investigator's Global Assessment [IGA] ≥ 2) were randomized to switch to guselkumab or continue ustekinumab. This post-hoc analysis evaluated the patient subgroup with residual mild psoriasis (IGA = 2) after initial ustekinumab therapy. Outcomes assessed included the Psoriasis Area and Severity Index (PASI), Dermatology Life Quality Index (DLQI), and Psoriasis Symptoms and Signs Diary (PSSD). Initially, 871 patients received ustekinumab. At Week 16, 161 randomized patients had residual mild psoriasis (IGA = 2). Among guselkumab- vs ustekinumab-treated patients at Week 28, 59.0% vs 27.7% achieved PASI 90, and 50.0% vs 21.0% achieved DLQI 0/1. Mean changes from baseline in PSSD score were -44 vs -28 and -50 vs -32, respectively, with thresholds of -40 considered clinically meaningful. Mean changes in PSSD itch score were -4.6 vs -2.9, with reductions ≥ 4.0 considered clinically meaningful. Treatment differences were maintained/increased through Week 52. Among patients with residual mild psoriasis after 16 weeks of ustekinumab, those switching to guselkumab had greater improvements in skin clearance, health-related quality of life, and patient-reported symptoms and signs than those continuing ustekinumab.
Conflict of interest statement
EE has worked as a consultant for AbbVie, Amgen, and Janssen. PW has carried out clinical trials for and/or has received honoraria as a consultant and/or speaker from AbbVie, Almirall, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen, LEO, Merck Sharp & Dohme, Novartis, Pfizer, Sandoz, and UCB. SK has received support for research from Bristol Myers Squibb, Celgene, LEO, Pfizer, and Takeda; as a speaker from AbbVie, Arcutis, Bristol Myers Squibb, Eli Lilly, Janssen, LEO, Pfizer, Regeneron, Sanofi, UCB; and as a consultant for AbbVie, Eli Lilly, Janssen, Regeneron, Sanofi, and UCB. PG was an employee of Janssen-Cilag Ltd of Johnson & Johnson at the time of this research; MG and JJ are employees of Janssen Research & Development, LLC of Johnson & Johnson; CH is an employee of Janssen Global Services of Johnson & Johnson; employees own stock/stock options in Johnson & Johnson, of which Janssen is a subsidiary. BK has received research support from/was a principal investigator (clinical trials) for AbbVie, Almirall, Janssen, Merck Sharpe & Dohme, Moonlake, Novartis, Pfizer, and UCB; been a consultant for AbbVie, Almirall, Celgene, Janssen, MC2 Therapeutics, Merck Sharpe & Dohme, Moonlake, Novartis, Pfizer, UCB, and Union; received honoraria from AbbVie, Almirall, Celgene, Janssen, Lilly, MC2 therapeutics, Moonlake, Novartis, Pfizer, Union, and UCB; and been on scientific advisory boards for AbbVie, Almirall, Celgene, GlaxoSmithKline, Janssen, Lilly, MC2 Therapeutics, Moonlake, Novartis, Pfizer, and UCB.
Figures


References
-
- Armstrong AW, Robertson AD, Wu J, Schupp C, Lebwohl MG. Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: findings from the National Psoriasis Foundation surveys, 2003–2011. JAMA Dermatol 2013; 149: 1180–1185. 10.1001/jamadermatol.2013.5264 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

