Efficacy and safety of different doses of vamorolone in boys with Duchenne muscular dystrophy: a systematic review and network meta-analysis
- PMID: 39233679
- PMCID: PMC11371629
- DOI: 10.3389/fneur.2024.1456559
Efficacy and safety of different doses of vamorolone in boys with Duchenne muscular dystrophy: a systematic review and network meta-analysis
Abstract
Background and objectives: Several recent clinical studies have indicated that vamorolone is comparable in effectiveness to glucocorticosteroids for treating Duchenne muscular dystrophy (DMD). However, there is a lack of extensive data regarding the efficacy and safety of various doses of vamorolone. We conducted a study to evaluate the efficacy of different doses of vamorolone in boys with DMD, and compare the safety of vamorolone vs. glucocorticosteroids, prednisone or deflazacort in boys with DMD.
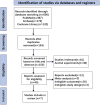
Methods: We performed systematic searches of the PubMed, Embase, and Cochrane Library databases for vamorolone, glucocorticosteroids, prednisone or deflazacort in boys with DMD. We assessed statistical heterogeneity across trials based on the Newcastle Ottawa scale (NOS) tool test and I2 values, and mean differences were pooled using the random-effects model. We used traditional meta-analysis to evaluate efficacy and safety of vamorolone 6.0 mg/kg/d vs. vamorolone 2.0 mg/kg/d and vamorolone vs. prednisone. A network meta-analysis was applied to estimated the safety of vamorolone in comparison to glucocorticosteroids, prednisone and deflazacort. Our meta-analysis were performed using Revman 5.4 software, and our network meta-analysis were performed using Stata/MP 18.0.
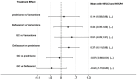
Results: In the meta-analysis, a total of 193 patients were analyzed across four clinical trials (97 patients receiving vamorolone 2 mg/kg per day; 96 patients receiving vamorolone 2 mg/kg per day). We observed that there were statistically significant differences in boys with DMD between vamorolone 6.0 mg/kg/d and vamorolone 2.0 mg/kg/d in TTSTANDV (MD = 0.03, 95%CI = 0.00-0.06, p = 0.04), TTRWV (MD = 0.13, 95%CI = 0.08-0.19, p < 0.01), 6MWT (MD = 24.54, 95%CI = 4.46-44.82, p = 0.02), TTCLIMBV (MD = 0.04, 95%CI = 0.01-0.06, p = 0.009), no significant difference in BMI z score (MD = 0.09, 95%CI = -0.03-0.20, p = 0.13). Indirect comparisons derived from network meta-analysis did not show significant differences among vamorolone, glucocorticosteroids, prednisone and deflazacort in BMI z score.
Conclusion: Our findings implied that boys with DMD who took vamorolone 6 mg/kg daily instead of 2 mg/kg daily may be safer and have superior motor function. However, more large sample randomized controlled trials are needed to confirm our results.
Systematic review registration: This systematic review and meta-analysis has been registered in the International Prospective Register of Ongoing Systematic Reviews PROSPERO (registration number: CRD42024562916).
Keywords: Duchenne muscular dystroph; deflazacort; glucocorticosteroids; prednisone; vamorolone.
Copyright © 2024 Wang, Zeng, Jiao, He, Li, Guo and Song.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

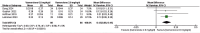
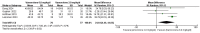
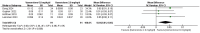
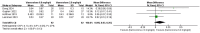

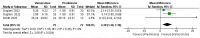
References
Publication types
LinkOut - more resources
Full Text Sources

