Improving Downstream Process Related Manufacturability Based on Protein Engineering-A Feasibility Study
- PMID: 39233725
- PMCID: PMC11369322
- DOI: 10.1002/elsc.202400019
Improving Downstream Process Related Manufacturability Based on Protein Engineering-A Feasibility Study
Abstract
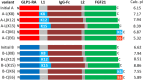
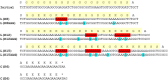
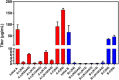
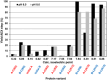
While bioactivity and a favorable safety profile for biotherapeutics is of utmost importance, manufacturability is also worth of consideration to ease the manufacturing process. Manufacturability in the scientific literature is mostly related to stability of formulated drug substances, with limited focus on downstream process-related manufacturability, that is, how easily can a protein be purified. Process-related impurities or biological impurities like viruses and host cell proteins (HCP) are present in the harvest which have mostly acid isoelectric points and need to be removed to ensure patient safety. Therefore, during molecule design, the surface charge of the target molecule should preferably differ sufficiently from the surface charge of the impurities to enable an efficient purification strategy. In this feasibility study, we evaluated the possibility of improving manufacturability by adapting the surface charge of the target protein. We generated several variants of a GLP1-receptor-agonist-Fc-domain-FGF21-fusion protein and demonstrated proof of concept exemplarily for an anion exchange chromatography step which then can be operated at high pH values with maximal product recovery allowing removal of HCP and viruses. Altering the surface charge distribution of biotherapeutic proteins can thus be useful allowing for an efficient manufacturing process for removing HCP and viruses, thereby reducing manufacturing costs.
Keywords: Fc‐fusion protein; anion exchange chromatography; codon usage; manufacturability; protein surface charge.
© 2024 Sanofi‐Aventis Deutschland GmbH. Engineering in Life Sciences published by Wiley‐VCH GmbH.
Figures


Similar articles
-
Demonstration of robust host cell protein clearance in biopharmaceutical downstream processes.Biotechnol Prog. 2008 May-Jun;24(3):615-22. doi: 10.1021/bp070396j. Epub 2008 Apr 15. Biotechnol Prog. 2008. PMID: 18410156
-
The prevention of an anomalous chromatographic behavior and the resulting successful removal of viruses from monoclonal antibody with an asymmetric charge distribution by using a membrane adsorber in highly efficient, anion-exchange chromatography in flow-through mode.Biotechnol Prog. 2020 May;36(3):e2955. doi: 10.1002/btpr.2955. Epub 2020 Jan 21. Biotechnol Prog. 2020. PMID: 31894893
-
Design and optimization of membrane chromatography for monoclonal antibody charge variant separation.Biotechnol Prog. 2022 Nov;38(6):e3288. doi: 10.1002/btpr.3288. Epub 2022 Jul 20. Biotechnol Prog. 2022. PMID: 35818846 Free PMC article.
-
Methods for addressing host cell protein impurities in biopharmaceutical product development.Biotechnol J. 2023 Mar;18(3):e2200115. doi: 10.1002/biot.202200115. Epub 2022 Dec 13. Biotechnol J. 2023. PMID: 36427352 Review.
-
Effective strategies for host cell protein clearance in downstream processing of monoclonal antibodies and Fc-fusion proteins.Protein Expr Purif. 2017 Jun;134:96-103. doi: 10.1016/j.pep.2017.04.006. Epub 2017 Apr 13. Protein Expr Purif. 2017. PMID: 28414067 Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous
