Human immunodeficiency virus-associated Lymphomas: EHA-ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up
- PMID: 39233903
- PMCID: PMC11369492
- DOI: 10.1002/hem3.150
Human immunodeficiency virus-associated Lymphomas: EHA-ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up
Abstract
This EHA-ESMO Clinical Practice Guideline provides key recommendations for managing HIV-associated lymphomas.The guideline covers clinical, imaging and pathological diagnosis; staging and risk assessment; treatment and follow-up.The author group encompasses a multidisciplinary group of experts from different institutions and countries in Europe.Recommendations are based on available scientific data and the authors' collective expert opinion.
© 2024 The Authors. Published by John Wiley & Sons Limited on behalf of European Hematology Association and by Elsevier Limited on behalf of European Society for Medical Oncology.
Conflict of interest statement
KH reports personal fees for advisory board membership from Gilead, Hexal, Incyte, Miltenyi, Novartis, Recordati and Roche; personal fees as an invited speaker from BeiGene, Bristol Myers Squibb (BMS), Novartis, Recordati, Roche and Servier; personal fees for a writing engagement from Hexal; institutional fees as coordinating Principal Investigator (PI) from Incyte and Roche; a non‐renumerated role as working group chair of the German Society of Hematology and Medical Oncology (DGHO); non‐remunerated advisory role for the European Hematology Association (EHA) Education Committee; and a non‐remunerated leadership role for the German Lymphoma Alliance. MB reports personal fees as an invited speaker from BMS, EUSA Pharma, Gilead, Janssen, Merck and ViiV Healthcare. IA reports personal fees for advisory board membership from AbbVie, AstraZeneca, Eli Lilly, Genesis/Incyte, Janssen, Novartis, Roche, Sobi and Takeda; personal fees as an invited speaker from AbbVie, AstraZeneca, Eli Lilly, Genesis/Incyte, Janssen, Novartis, Roche, Sandoz, Sobi and Takeda; non‐remunerated leadership roles for the Croatian Cooperative Group for Hematologic Diseases and the EHA Lymphoma Specialized Working Group; and non‐renumerated membership of the Board of Directors of the Croatian Hematological Society and the European Lymphoma Institute. MBO reports personal fees for advisory board participation from Kite Pharma, Novartis and Roche; personal fees as an invited speaker from Janssen, Kite Pharma, Roche and Takeda; a personal and institutional research grant from Kite Pharma (Gilead/Kite Pharma award); and non‐renumerated leadership roles for the Spanish Lymphoma Group (GELTAMO; coordinator of the Aggressive Group and member of the Scientific Committee), the Madrid Hematology Society (member of the Board of Directors, treasurer) and the Spanish Society of Hematology and Hemotherapy (member of the Board of Directors, accountant). CBe reports personal fees for expert testimony from Janssen. UB reports personal fees for advisory board membership from Janssen‐Cilag; personal fees as an invited speaker from AstraZeneca and Janssen‐Cilag; personal fees for writing engagements from AbbVie and AstraZeneca; travel grants from AbbVie, BeiGene and Gilead; compensation for congress registration fees from Lilly; a non‐renumerated role as PI for Regeneron and Roche; and non‐renumerated membership of DGHO, the German Cancer Society (DKG) and EHA. CC reports no conflicts of interest. SC reports employment as a treatment advocate by the charity HIV i‐Base. KC reports personal fees for advisory board membership from AbbVie, Atara, Celgene, Incyte, Janssen, Kite, Roche and Takeda; personal fees as an invited speaker and personal travel grants/conference support from Celgene, Incyte, Kite, Roche and Takeda; personal fees for consulting from Atara, Celgene, Incyte and Kite. ADP reports institutional fees as an invited speaker from Gilead (non‐promotional teaching). MH reports personal fees for advisory board membership from Amgen, EUSA Pharma, Sanofi, Stemline and Takeda; and personal fees as an invited speaker from EUSA Pharma, Gilead, Janssen, Jazz Pharmaceuticals and Sanofi. CH reports personal fees for advisory board membership and as an invited speaker from EUSA Pharma, Gilead Sciences, Janssen‐Cilag, MSD and ViiV Healthcare; and institutional fees as local PI (for clinical studies conducted at institution) from Janssen‐Cilag, MSD and ViiV Healthcare. MJK reports institutional fees for advisory board membership from Adicet Bio, BMS/Celgene, Galapagos, Kite (a Gilead Company), Miltenyi Biotec and Novartis; institutional fees as an invited speaker from BeiGene, BMS/Celgene, Kite (a Gilead Company) and Novartis; institutional fees as local PI from BMS/Celgene; institutional fees as coordinating PI from Galapagos, Kite (a Gilead Company), Miltenyi Biotec and Novartis, and an institutional travel grant from AbbVie. SM reports personal fees for participation in a Data Monitoring Committee from Bayer. JTN reports personal fees for advisory board membership from Recordati Rare Diseases EUSA Pharma; personal fees as an invited speaker from Novartis and Recordati Rare Diseases EUSA Pharma; a personal research grant from Gilead Spain; and institutional funding from Recordati Rare Diseases EUSA Pharma. EO reports personal fees as an expert for grant attributions from CSL Behring; personal fees as a consultant from EUSA Pharma; an institutional research grant on unicentric Castleman disease pathogenesis from the Castleman Disease Collaborative Network (CDCN); and non‐renumerated membership of the advisory board of CDCN. AR reports personal fees for advisory board membership from Incyte, Italfarmaco and Takeda; personal fees as an expert discussant from Janssen and Servier; personal fees as an invited speaker from Sobi and Takeda; institutional fees as local PI from MSD, Pharmacyclis and Sanofi. JMR reports personal fees for advisory board membership from Amgen, Pfizer, Shire and Takeda; personal fees as an invited speaker from Amgen; and personal fees as local PI from Takeda. PS reports personal fees as an invited speaker from MSD and ViiV Healthcare; and a personal and institutional research grant from Gilead Sciences. BvT reports personal fees for advisory and consultancy roles for Allogene, Amgen, BMS/Celgene, Cerus, Gilead Kite, Incyte, IQVIA, Janssen‐Cilag GmbH, Lilly, Miltenyi, MSD, Noscendo, Novartis, Pentixapharm, Pfizer, Pierre Fabre, QualWorld, Roche, Sobi and Takeda; personal fees as an invited speaker from AbbVie, AstraZeneca, BMS/Celgene, Gilead Kite, Incyte, Lilly, MSD, Novartis, Roche Pharma AG and Takeda; institutional funding from Esteve, MSD, Novartis and Takeda; and travel support from AbbVie, AstraZeneca, Gilead Kite, Lilly, MSD, Pierre Fabre, Roche, Takeda and Novartis. CBu reports personal fees for advisory board membership from AbbVie, BeiGene, Celltrion, Gilead Sciences, Incyte, Janssen, Lilly Deutschland GmbH, MorphoSys, Novartis, Pfizer, Regeneron, Roche and Sobi; personal fees as an invited speaker from AbbVie, BeiGene, Celltrion, Gilead Sciences, Incyte, Janssen, Lilly Deutschland GmbH, MorphoSys, Novartis, Pfizer, Regeneron, Roche and Sobi; and institutional funding from AbbVie Amgen, Bayer, Celltrion, Janssen, MSD, Pfizer and Roche (all for investigator‐sponsored clinical trials and registries). MD reports personal fees as an advisory board member from AbbVie, AstraZeneca, BeiGene, BMS/Celgene, Gilead, Janssen, Lilly/Loxo, Novartis and Roche; personal fees as an invited speaker for AstraZeneca, BeiGene, Gilead/Kite, Janssen, Lilly, Novartis and Roche; institutional research grants from AbbVie, Bayer, Celgene, Janssen, Lilly and Roche; institutional funding from Gilead/Kite; and non‐renumerated membership of the American Society of Clinical Oncology, American Society of Hematology (subcommittee), DGHO (prior Board member), EHA (Executive Board), ESMO (Faculty) and the Lymphoma Research Foundation (Mantle Cell Lymphoma Consortium). AD reports personal fees as an advisory board member for AbbVie, Acerta Pharma, AstraZeneca, BMS/Celgene, Genmab, Gilead, Incyte, Karyopharm, Kite Pharma, Regeneron, Roche, Sobi and Takeda; personal fees as an invited speaker for AstraZeneca, Gilead and Roche; institutional research grants for conduct of commercial research and funding of IST from Acerta Pharma and Roche; institutional research grants for conduct of commercial research from ADC Therapeutics, AstraZeneca, BMS/Celgene, Gilead and Pfizer; institutional research grant from MSD (no financial interest); non‐renumerated leadership role, member and UK Board representative for the Precision Medicine in Aggressive Lymphoma Consortium of the International Extranodal Lymphoma Study Group; advisory role and international advisor for the Swiss SAKK Lymphoma Project Group and member of the UK National Cancer Research Institute's High Grade Lymphoma Study Group.
Figures
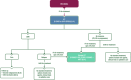
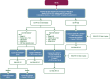
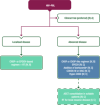
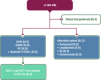
References
-
- Poizot‐Martin I, Lions C, Allavena C, et al. Spectrum and incidence trends of AIDS‐ and non‐AIDS‐defining cancers between 2010 and 2015 in the French Dat'AIDS cohort. Cancer Epidemiol Biomarkers Prev. 2021;30(3):554‐563. - PubMed
-
- Carbone A, Vaccher E, Gloghini A. Hematologic cancers in individuals infected by HIV. Blood. 2022;139(7):995‐1012. - PubMed
LinkOut - more resources
Full Text Sources
