A review of the fernane-type triterpenoids as anti-fungal drugs
- PMID: 39234110
- PMCID: PMC11371599
- DOI: 10.3389/fphar.2024.1447450
A review of the fernane-type triterpenoids as anti-fungal drugs
Abstract
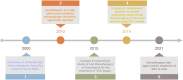
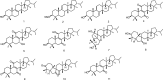
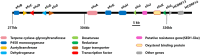
Human fungal pathogens could cause a broad plethora of infections in both the immunocompetent and immunocompromised host. Fungal infections have become important causes of morbidity and mortality in recent years, the current arsenal of anti-fungal therapies was restricted. Ibrexafungerp was a novel, highly bioavailable glucan synthase inhibitor formulated for both intravenous and oral administration being developed by Scynexis; it was also the first novel anti-fungal drug class approved in more than 20 years. Ibrexafungerp was one semi-synthetic derivative of enfumafungin, a natural product isolated from fungi. This review reported the discovery of enfumafungin and ibrexafungerp, their anti-fungal mechanism, summed up 63 fernane-type triterpenoids from natural products, including 49 from plants, 9 from fungi and 5 from lichen. In addition, the review summarized the progress of enzymes responsible for the biosynthesis of type II fernane triterpenoid (enfumafungin skeleton) and type I fernane triterpenoid (polytolypin skeleton). The good example kept our confidence up for searching for new leading compounds and discovering drugs from fungi.
Keywords: anti-fungal; enfumafungin; fernane type; ibrexafungerp; triterpenoid.
Copyright © 2024 Liu, Zhang, Liu, Lu, Li and Pei.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Aly A. H., Debbab A., Proksch P. (2011). Fifty years of drug discovery from fungi. Fungal Divers. 50, 3–19. 10.1007/s13225-011-0116-y - DOI
Publication types
LinkOut - more resources
Full Text Sources

