The role of pituitary adenylate cyclase-activating polypeptide neurons in the hypothalamic ventromedial nucleus and the cognate PAC1 receptor in the regulation of hedonic feeding
- PMID: 39234295
- PMCID: PMC11371718
- DOI: 10.3389/fnut.2024.1437526
The role of pituitary adenylate cyclase-activating polypeptide neurons in the hypothalamic ventromedial nucleus and the cognate PAC1 receptor in the regulation of hedonic feeding
Abstract
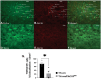
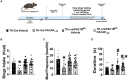
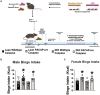
Obesity is a health malady that affects mental, physical, and social health. Pathology includes chronic imbalance between energy intake and expenditure, likely facilitated by dysregulation of the mesolimbic dopamine (DA) pathway. We explored the role of pituitary adenylate cyclase-activating polypeptide (PACAP) neurons in the hypothalamic ventromedial nucleus (VMN) and the PACAP-selective (PAC1) receptor in regulating hedonic feeding. We hypothesized that VMN PACAP neurons would inhibit reward-encoding mesolimbic (A10) dopamine neurons via PAC1 receptor activation and thereby suppress impulsive consumption brought on by intermittent exposure to highly palatable food. Visualized whole-cell patch clamp recordings coupled with in vivo behavioral experiments were utilized in wildtype, PACAP-cre, TH-cre, and TH-cre/PAC1 receptor-floxed mice. We found that bath application of PACAP directly inhibited preidentified A10 dopamine neurons in the ventral tegmental area (VTA) from TH-cre mice. This inhibitory action was abrogated by the selective knockdown of the PAC1 receptor in A10 dopamine neurons. PACAP delivered directly into the VTA decreases binge feeding accompanied by reduced meal size and duration in TH-cre mice. These effects are negated by PAC1 receptor knockdown in A10 dopamine neurons. Additionally, apoptotic ablation of VMN PACAP neurons increased binge consumption in both lean and obese, male and female PACAP-cre mice relative to wildtype controls. These findings demonstrate that VMN PACAP neurons blunt impulsive, binge feeding behavior by activating PAC1 receptors to inhibit A10 dopamine neurons. As such, they impart impactful insight into potential treatment strategies for conditions such as obesity and food addiction.
Keywords: A10 dopamine neurons; PAC1 receptor; food addiction; hedonic feeding; hypothalamic ventromedial nucleus; pituitary adenylate cyclase-activating polypeptide; ventral tegmental area.
Copyright © 2024 Sayers, Le and Wagner.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials

