Characterization of AAV vectors: A review of analytical techniques and critical quality attributes
- PMID: 39234444
- PMCID: PMC11372808
- DOI: 10.1016/j.omtm.2024.101309
Characterization of AAV vectors: A review of analytical techniques and critical quality attributes
Abstract
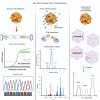
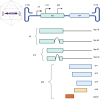
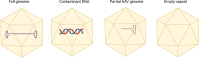
Standardized evaluation of adeno-associated virus (AAV) vector products for biotherapeutic application is essential to ensure the safety and efficacy of gene therapies. This includes analyzing the critical quality attributes of the product. However, many of the current analytical techniques used to assess these attributes have limitations, including low throughput, large sample requirements, poorly understood measurement variability, and lack of comparability between methods. To address these challenges, it is essential to establish higher-order reference methods that can be used for comparability measurements, optimization of current assays, and development of reference materials. Highly precise methods are necessary for measuring the empty/partial/full capsid ratios and the titer of AAV vectors. Additionally, it is important to develop methods for the measurement of less-established critical quality attributes, including post-translational modifications, capsid stoichiometry, and methylation profiles. By doing so, we can gain a better understanding of the influence of these attributes on the quality of the product. Moreover, quantification of impurities, such as host-cell proteins and DNA contaminants, is crucial for obtaining regulatory approval. The development and application of refined methodologies will be essential to thoroughly characterize AAV vectors by informing process development and facilitating the generation of reference materials for assay validation and calibration.
Keywords: AAV; CQAs; analytical methodologies; capsid identity; characterization; critical quality attributes; gene therapy; genetic identity; viral vector.
Crown Copyright © 2024 Published by Elsevier Inc. on behalf of The American Society of Gene and Cell Therapy.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- U.S. Food and Drug Administration (FDA) Human gene therapy for neurodegenerative diseases guidance for industry. 2022. https://www.fda.gov/regulatory-information/search-fda-guidance-documents...
-
- European Medicines Agency (EMA) Guideline on development, production, characterisation and specifications for monoclonal antibodies and related products, EMEA/CHMP/BWP/532517/2008. 2016. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-de...
Publication types
LinkOut - more resources
Full Text Sources

