Comparative study of systemic and local delivery of mesenchymal stromal cells for the treatment of chronic kidney disease
- PMID: 39234562
- PMCID: PMC11373351
- DOI: 10.3389/fcell.2024.1456416
Comparative study of systemic and local delivery of mesenchymal stromal cells for the treatment of chronic kidney disease
Abstract
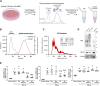
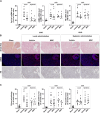
Renal fibrosis, characterized by excessive extracellular matrix accumulation, leads to a progressive decline of renal function and is a common endpoint of chronic kidney disease (CKD). Current treatments primarily focus on managing underlying diseases, offering limited direct intervention for the fibrotic process. This study explores the anti-fibrotic potential of human adipose-derived mesenchymal stromal cells (MSCs) and their derived extracellular vesicles (EVs) in the context of CKD, emphasizing the effects of systemic versus local delivery methods. Preconditioned MSCs (Pr-MSCs) were treated with TNF-α and IFN-γ to enhance their immunomodulatory capabilities, and demonstrated significant anti-fibrotic effects in vitro, reducing mRNA expression of fibrosis markers in TGF-β stimulated HKC-8 cells. Our in vivo findings from a murine unilateral ureteral obstruction (UUO) model of CKD showed that local deliveries of Pr-MSCs reduced collagen deposition and increased expression of the anti-inflammatory cytokine IL-10. Systemic administration of Pr-MSCs did not show any significant effect on UUO-induced injury. In addition, EVs did not replicate the anti-fibrotic effects observed with their parent cells, suggesting that soluble proteins or metabolites secreted by Pr-MSCs might be the primary mediators of the anti-fibrotic and immunomodulatory effects. This study provides critical insights into the therapeutic efficacy of MSCs, highlighting the importance of delivery methods and the potential of preconditioning strategies in enhancing MSC-based therapies for renal fibrosis.
Keywords: chronic kidney disease; delivery; fibrosis; inflammation; mesenchymal stem cells; mesenchymal stromal cells (MSCs).
Copyright © 2024 Gregersen, Kresse, Atay, Boysen, Nejsum, Eijken and Nørregaard.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Aabling R. R., Alstrup T., Kjær E. M., Poulsen K. J., Pedersen J. O., Revenfeld A. L., et al. (2023). Reconstitution and post-thaw storage of cryopreserved human mesenchymal stromal cells: pitfalls and optimizations for clinically compatible formulants. Regen. Ther. 23, 67–75. 10.1016/j.reth.2023.03.006 - DOI - PMC - PubMed
-
- Almeida A., Lira R., Oliveira M., Martins M., Azevedo Y., Silva K. R., et al. (2022). Bone marrow-derived mesenchymal stem cells transplantation ameliorates renal injury through anti-fibrotic and anti-inflammatory effects in chronic experimental renovascular disease. Biomed. J. 45 (4), 629–641. 10.1016/j.bj.2021.07.009 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

