Experimental TET2 Clonal Hematopoiesis Predisposes to Renal Hypertension Through an Inflammasome-Mediated Mechanism
- PMID: 39234670
- PMCID: PMC11519839
- DOI: 10.1161/CIRCRESAHA.124.324492
Experimental TET2 Clonal Hematopoiesis Predisposes to Renal Hypertension Through an Inflammasome-Mediated Mechanism
Abstract
Background: Hypertension incidence increases with age and represents one of the most prevalent risk factors for cardiovascular disease. Clonal events in the hematopoietic system resulting from somatic mutations in driver genes are prevalent in elderly individuals who lack overt hematologic disorders. This condition is referred to as age-related clonal hematopoiesis (CH), and it is a newly recognized risk factor for cardiovascular disease. It is not known whether CH and hypertension in the elderly are causally related and, if so, what are the mechanistic features.
Methods: A murine model of adoptive bone marrow transplantation was employed to examine the interplay between Tet2 (ten-eleven translocation methylcytosine dioxygenase 2) clonal hematopoiesis and hypertension.
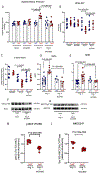
Results: In this model, a subpressor dose of Ang II (angiotensin II) resulted in elevated systolic and diastolic blood pressure as early as 1 day after challenge. These conditions led to the expansion of Tet2-deficient proinflammatory monocytes and bone marrow progenitor populations. Tet2 deficiency promoted renal CCL5 (C-C motif ligand 5) chemokine expression and macrophage infiltration into the kidney. Consistent with macrophage involvement, Tet2 deficiency in myeloid cells promoted hypertension when mice were treated with a subpressor dose of Ang II. The hematopoietic Tet2-/- condition led to sodium retention, renal inflammasome activation, and elevated levels of IL (interleukin)-1β and IL-18. Analysis of the sodium transporters indicated NCC (sodium-chloride symporter) and NKCC2 (Na+-K+-Cl- cotransporter 2) activation at residues Thr53 and Ser105, respectively. Administration of the NLRP3 (NLR family pyrin domain containing 3) inflammasome inhibitor MCC950 reversed the hypertensive state, sodium retention, and renal transporter activation.
Conclusions: Tet2-mediated CH sensitizes mice to a hypertensive stimulus. Mechanistically, the expansion of hematopoietic Tet2-deficient cells promotes hypertension due to elevated renal immune cell infiltration and activation of the NLRP3 inflammasome, with consequences on sodium retention. These data indicate that carriers of TET2 CH could be at elevated risk for the development of hypertension and that immune modulators could be useful in treating hypertension in this patient population.
Keywords: clonal hematopoiesis; hypertension; inflammation; kidney; sodium.
Conflict of interest statement
None.
Figures





Comment in
-
Salt and CHIP: Tet2-CH Aggravates Salt-Sensitive Hypertension in Mice.Circ Res. 2024 Oct 11;135(9):951-953. doi: 10.1161/CIRCRESAHA.124.325364. Epub 2024 Oct 10. Circ Res. 2024. PMID: 39388535 Free PMC article. No abstract available.
References
-
- Colby SL OJ. Projections of the Size and Composition of the U.S. Population (2014 to 2060). 2015.
-
- Martin SS, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker-Smith CM, Barone Gibbs B, Beaton AZ, Boehme AK, et al. American Heart Association Council on E, Prevention Statistics C, Stroke Statistics S. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation. 2024;149:e347–e913. - PMC - PubMed
-
- Prevention CfDCa. Hypertension Cascade: Hypertension Prevalence, Treatment and Control Estimates Among U.S. Adults Aged 18 Years and Older Applying the Criteria from the American College of Cardiology and American Heart Association’s 2017 Hypertension Guideline—NHANES 2017–2020.. 2023;2023.
-
- Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

