Drug Target to Alleviate Mitochondrial Dysfunctions in Alzheimer's Disease: Recent Advances and Therapeutic Implications
- PMID: 39234772
- PMCID: PMC11333791
- DOI: 10.2174/1570159X22666240426091311
Drug Target to Alleviate Mitochondrial Dysfunctions in Alzheimer's Disease: Recent Advances and Therapeutic Implications
Abstract
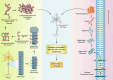
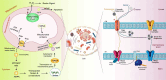
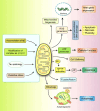
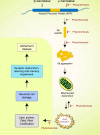
Alzheimer's disease (AD) is a severe progressive neurodegenerative condition associated with neuronal damage and reduced cognitive function that primarily affects the aged worldwide. While there is increasing evidence suggesting that mitochondrial dysfunction is one of the most significant factors contributing to AD, its accurate pathobiology remains unclear. Mitochondrial bioenergetics and homeostasis are impaired and defected during AD pathogenesis. However, the potential of mutations in nuclear or mitochondrial DNA encoding mitochondrial constituents to cause mitochondrial dysfunction has been considered since it is one of the intracellular processes commonly compromised in early AD stages. Additionally, electron transport chain dysfunction and mitochondrial pathological protein interactions are related to mitochondrial dysfunction in AD. Many mitochondrial parameters decline during aging, causing an imbalance in reactive oxygen species (ROS) production, leading to oxidative stress in age-related AD. Moreover, neuroinflammation is another potential causative factor in AD-associated mitochondrial dysfunction. While several treatments targeting mitochondrial dysfunction have undergone preclinical studies, few have been successful in clinical trials. Therefore, this review discusses the molecular mechanisms and different therapeutic approaches for correcting mitochondrial dysfunction in AD, which have the potential to advance the future development of novel drug-based AD interventions.
Keywords: Alzheimer’s disease; ROS.; drug target; mitochondria; mitochondrial dysfunction; therapeutic approaches.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Conflict of interest statement
The authors declare no conflict of interest, financial or otherwise.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
