Targeting Dual Immune Checkpoints PD-L1 and HLA-G by Trispecific T Cell Engager for Treating Heterogeneous Lung Cancer
- PMID: 39234811
- PMCID: PMC11538689
- DOI: 10.1002/advs.202309697
Targeting Dual Immune Checkpoints PD-L1 and HLA-G by Trispecific T Cell Engager for Treating Heterogeneous Lung Cancer
Abstract
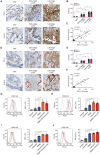
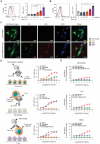
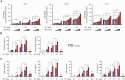
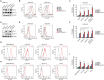
Immunotherapy targeting immune checkpoints (ICPs), such as programmed death-ligand-1 (PD-L1), is used as a treatment option for advanced or metastatic non-small cell lung cancer (NSCLC). However, overall response rate to anti-PD-L1 treatment is limited due to antigen heterogeneity and the immune-suppressive tumor microenvironment. Human leukocyte antigen-G (HLA-G), an ICP as well as a neoexpressed tumor-associated antigen, is previously demonstrated to be a beneficial target in combination with anti-PD-L1. In this study, a nanobody-based trispecific T cell engager (Nb-TriTE) is developed, capable of simultaneously binding to T cells, macrophages, and cancer cells while redirecting T cells toward tumor cells expressing PD-L1- and/or HLA-G. Nb-TriTE shows broad spectrum anti-tumor effects in vitro by augmenting cytotoxicity mediated by human peripheral blood mononuclear cells (PBMCs). In a humanized immunodeficient murine NSCLC model, Nb-TriTE exhibits superior anti-cancer potency compared to monoclonal antibodies and bispecific T cell engagers. Nb-TriTE, at the dose with pharmacoactivity, does not induce additional enhancement of circulating cytokines secretion from PMBCs. Nb-TriTE effectively prolongs the survival of mice without obvious adverse events. In conclusion, this study introduces an innovative therapeutic approach to address the challenges of immunotherapy and the tumor microenvironment in NSCLC through utilizing the dual ICP-targeting Nb-TriTE.
Keywords: human leukocyte antigen‐G (HLA‐G); immune checkpoint (ICP); nanobody‐based trispecific T cell engager (Nb‐TriTE); non‐small cell lung cancer (NSCLC); programmed death‐ligand 1 (PD‐L1).
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
Y.C.L., J.H.C.H., and H.C.C. are employed by Shine‐On BioMedical Co., Ltd. during the conduct of this study. C.C.H. and S.C.C. are employed and owned equity interests in Shine‐On BioMedical Co., Ltd. during the conduct of this study. The other authors declare no direct conflicts of interest.
Figures


References
-
- a) Pennock G. K., Chow L. Q., Oncologist 2015, 20, 812; - PMC - PubMed
- b) Marshall H. T., Djamgoz M. B. A., Front Oncol 2018, 8, 315; - PMC - PubMed
- c) Jafari S., Molavi O., Kahroba H., Hejazi M. S., Maleki‐Dizaji N., Barghi S., Kiaie S. H., Jadidi‐Niaragh F., Cell. Mol. Life Sci. 2020, 77, 3693; - PMC - PubMed
- d) Ogasawara K., Newhall K., Maxwell S. E., Dell'Aringa J., Komashko V., Kilavuz N., Delarue R., Czuczman M., Sternas L., Rose S., Beach C. L., Novick S., Zhou S., Palmisano M., Li Y., Clin. Pharmacokinet. 2020, 59, 217. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- MOST-110-2314-B-039-052/Ministry of Science and Technology/National Science and Technology Council
- MOST-110-2314-B-039-053/Ministry of Science and Technology/National Science and Technology Council
- MOST-111-2321-B-039-006/Ministry of Science and Technology/National Science and Technology Council
- MOST-109-2314-B-039-047-MY3/Ministry of Science and Technology/National Science and Technology Council
- NSCT-112-2314-B-039-053/Ministry of Science and Technology/National Science and Technology Council
LinkOut - more resources
Full Text Sources
Medical
Research Materials
