Investigation of health care use and a possible prodrome before the first attack in NMOSD and MOGAD
- PMID: 39234853
- PMCID: PMC11457589
- DOI: 10.1177/13524585241272939
Investigation of health care use and a possible prodrome before the first attack in NMOSD and MOGAD
Abstract
Background: Prodromal phases are well recognized in many inflammatory and neurodegenerative diseases, including multiple sclerosis. We evaluated the possibility of a prodrome in aquaporin-4 antibody positive (AQP4+) neuromyelitis optica spectrum disorder (NMOSD) and myelin oligodendrocyte glycoprotein antibody disease (MOGAD) using health administrative data.
Methods: We investigated individuals with AQP4 + NMOSD and MOGAD, confirmed by medical chart review, in Ontario, Canada. Each NMOSD and MOGAD participant was matched 1:5 to general population controls by sex, birth year, immigrant status, and region. Total outpatient visits and hospitalizations were compared in the 5 years preceding the incident attack in multivariable negative binomial models.
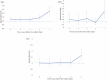
Results: We identified 96 people with AQP4 + NMOSD, matched to 479 controls, and 61 people with MOGAD, matched to 303 controls. In the 5 years preceding the incident attack, health care use was elevated for outpatient visits and hospitalizations for the NMOSD cohort (adjusted rate ratio (aRR): 1.47; 95% confidence interval (CI): 1.25-1.73; aRR: 1.67; 95% CI: 1.19-2.36, respectively) but not for MOGAD. Rate ratios steadily increased in NMOSD for outpatient visits in the 2 years preceding the incident attack.
Conclusion: Our findings support a prodromal phase preceding clinical onset of AQP4 + NMOSD. Earlier recognition and management of NMOSD patients may be possible.
Keywords: AQP4; MOGAD; NMOSD; prodrome.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: D.L.R. has received research funding from MS Canada, the National MS Society, CMSC, University of Toronto Division of Neurology, and Roche Canada. She has received speaker or consultant fees from Alexion, Biogen, EMD Serono, Horizon Therapeutics, Novartis, Roche, Sanofi Aventis, and Touch IME. M.S.F. has received research or educational grants from Sanofi-Genzyme Canada. He has received consulting fees or honoraria from Alexion/Astra Zeneca, Biogen Idec, EMD Inc./EMD Serono/Merck Serono, Find Therapeutics, Hoffman La-Roche, Horizon Therapeutics, Novartis, Quanterix, Sanofi-Genzyme, and Teva Canada Innovation. He has served as a member of a company advisory board, board of directors or other similar group for Alexion/Astra Zeneca, Actelion/Janssen (J&J), Atara Biotherapeutics, Bayer Healthcare, Celestra Health, EMD Inc./Merck Serono, Find Therapeutics, Hoffman La-Roche, Novartis, Sanofi-Genzyme, and Setpoint Medical. He has been part of a speaker’s bureau for Hoffman La-Roche, Novartis, and EMD Inc. A.K. has nothing to declare. L.L. is a site investigator for studies funded by Roche, Novartis, and Sanofi Aventis. He has received consultation fees from Alexion, Biogen, Bristol Myers Squibb, EMD Serono, Novartis, Roche, and Sanofi Aventis. J.L. has nothing to disclose. C.M. has received research support from MS Canada, the National MS Society, and the Canadian Institutes of Health Research (CIHR). S.A.M. has served as an advisory board member or received consulting fees from Biogen Idec, Bristol Myers Squibb/Celgene, EMD Serono, Novartis, Roche, Sanofi-Genzyme, and Teva Neurosciences. She has participated in a speaker’s bureau for Biogen Idec, Bristol Myers Squibb/Celgene, EMD Serono, Novartis, Roche, and Sanofi-Genzyme. She has received research support from Biogen Idec, Novartis, Roche, and Sanofi-Genzyme. She has participated as a site investigator in clinical trials sponsored by AbbVie, Bristol Myers Squibb/Celgene, EMD Serono, Novartis, Genzyme, Roche, and Sanofi-Genzyme. H.T. has received research support from the National Multiple Sclerosis Society, the Canadian Institutes of Health Research, the Multiple Sclerosis Society of Canada, the Multiple Sclerosis Scientific Research Foundation, and the EDMUS Foundation (“Fondation EDMUS contre la sclérose en plaques”). M.V.V. has received salary support as a co-investigator from National MS Society. R.A.M. receives research funding from CIHR, MS Canada, Crohn’s and Colitis Canada, National Multiple Sclerosis Society, CMSC, the Arthritis Society, and the US Department of Defense and is a co-investigator on studies receiving funding from Biogen Idec and Roche Canada. She holds the Waugh Family Chair in Multiple Sclerosis.
Figures
Comment in
-
Evidence for an NMOSD prodrome.Nat Rev Neurol. 2024 Nov;20(11):643. doi: 10.1038/s41582-024-01030-1. Nat Rev Neurol. 2024. PMID: 39402246 No abstract available.
References
-
- Marrie RA, Allegretta M, Barcellos LF, et al. From the prodromal stage of multiple sclerosis to disease prevention. Nat Rev Neurol 2022; 18(9): 559–572. - PubMed
-
- Wijnands JMA, Kingwell E, Zhu F, et al. Health-care use before a first demyelinating event suggestive of a multiple sclerosis prodrome: A matched cohort study. Lancet Neurol 2017; 16(6): 445–451. - PubMed
-
- Disanto G, Zecca C, MacLachlan S, et al. Prodromal symptoms of multiple sclerosis in primary care. Ann Neurol 2018; 83(6): 1162–1173. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

