Safety and efficacy of acalabrutinib plus bendamustine and rituximab in patients with treatment-naïve or relapsed/refractory mantle cell lymphoma: phase Ib trial
- PMID: 39234862
- PMCID: PMC11873691
- DOI: 10.3324/haematol.2023.284896
Safety and efficacy of acalabrutinib plus bendamustine and rituximab in patients with treatment-naïve or relapsed/refractory mantle cell lymphoma: phase Ib trial
Abstract
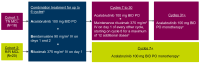
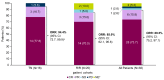
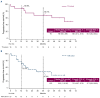
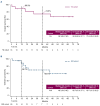
This multicenter, open-label, phase Ib study (ACE-LY-106) assessed the safety and efficacy of acalabrutinib, bendamustine, and rituximab (ABR) in treatment-naïve (TN) and relapsed or refractory (R/R) mantle cell lymphoma (MCL). Patients received acalabrutinib from cycle 1 until disease progression or treatment discontinuation, bendamustine on days 1 and 2 of each cycle for up to 6 cycles, and rituximab on day 1 of each cycle for 6 cycles, continuing every other cycle from cycle 8 for 12 additional doses (TN cohort). Eighteen patients enrolled in the TN cohort and 20 in the R/R cohort. Median duration of exposure to acalabrutinib was 34.0 and 14.6 months in the TN and R/R cohorts, respectively. No new safety risks were identified, and most adverse events (AE) were grades 1 or 2. Thirteen patients from the TN cohort (72.2%) and 17 patients from the R/R cohort (85.0%) reported grade 3-4 AE, most commonly neutropenia (TN: 38.9%; R/R: 50.0%). AE leading to death were pneumonitis (N=1, TN cohort), COVID-19, and cerebrospinal meningitis (N=1 each, R/R cohort). Overall response was 94.4% and 85.0% in the TN and R/R cohorts, respectively; complete response rates were 77.8% and 70.0%, respectively. After a median follow-up of 47.6 months, median progression-free survival (PFS) and overall survival (OS) were not reached in the TN cohort. After a median follow-up of 20.4 months, median PFS was 28.6 months and OS was not reached in the R/R cohort. Results indicate that ABR was safe and efficacious, supporting further study in patients with TN MCL.
Figures

References
-
- Dreyling M, Campo E, Hermine O, et al. . Newly diagnosed and relapsed mantle cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(Suppl 4):iv62-iv71. - PubMed
-
- Jain P, Wang ML. Mantle cell lymphoma in 2022--a comprehensive update on molecular pathogenesis, risk stratification, clinical approach, and current and novel treatments. Am J Hematol. 2022;97(5):638-656. - PubMed
-
- Robak T, Huang H, Jin J, et al. . Bortezomib-based therapy for newly diagnosed mantle-cell lymphoma. N Engl J Med. 2015;372(10):944-953. - PubMed
-
- Robak T, Jin J, Pylypenko H, et al. . Frontline bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) versus rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in transplantation-ineligible patients with newly diagnosed mantle cell lymphoma: final overall survival results of a randomised, open-label, phase 3 study. Lancet Oncol. 2018;19(11):1449-1458. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

