Lubricating drops for contact lens discomfort in adults
- PMID: 39234924
- PMCID: PMC11375780
- DOI: 10.1002/14651858.CD015751.pub2
Lubricating drops for contact lens discomfort in adults
Abstract
Background: Contact lens discomfort is a symptom-based clinical diagnosis that affects 13% to 75% of contact lens wearers. The Tear Film and Ocular Surface Society defines contact lens discomfort as "a condition characterized by episodic or persistent adverse ocular sensations related to lens wear either with or without visual disturbance, resulting from reduced compatibility between the lens and ocular environment, which can lead to decreased wearing time and discontinuation from lens wear." Signs of the condition include conjunctival hyperemia, corneal and conjunctival staining, altered blinking patterns, lid wiper epitheliopathy, and meibomian gland dysfunction. Eye care specialists often treat contact lens discomfort with lubricating drops, including saline, although there is no clear evidence showing this treatment is effective and safe.
Objectives: To evaluate the efficacy and safety of lubricating drops for ocular discomfort associated with contact lens wear in adults.
Search methods: We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register), MEDLINE, Embase.com, two other databases, and two trials registries to May 2024, without date or language restrictions.
Selection criteria: We included parallel-group randomized controlled trials (RCTs) that evaluated lubricating drops, including saline, versus no treatment, or that evaluated lubricating drops versus saline, in adult contact lens wearers. We included studies regardless of publication status, language, or year of publication.
Data collection and analysis: We applied standard Cochrane methodology. The critical outcome was contact lens discomfort. Important outcomes were corneal fluorescein staining and conjunctival redness. Adverse outcomes were incident microbial keratitis, inflammatory corneal infiltrates, and participant discontinuation. We assessed risk of bias for outcomes reported in the summary of findings table using the Cochrane risk of bias tool RoB 2, and we rated the certainty of the evidence using GRADE.
Main results: We included seven RCTs conducted in the USA, Canada, Italy, and France. They randomized a total of 463 participants to lubricating drops, saline, or no treatment. Four trials evaluated lubricating drops and saline versus no treatment, but one of them provided no usable outcome data. Three trials evaluated lubricating drops versus saline. Study characteristics All trial participants were adults, and the mean age ranged from 25.7 years to 36.7 years. The proportion of women varied from 15% to 82%. The trials lasted between one and four weeks. Of the five trials that reported contact lens discomfort, we judged three at high risk of bias, and considered the other two had some risk of bias concerns. Lubricating drops (including saline) versus no treatment Lubricating drops compared with no treatment may reduce contact lens discomfort, measured on a 37-point scale (lower is better), but the evidence is very uncertain (mean difference [MD] -5.9 points, 95% confidence interval [CI] -3.74 to -8.05; 2 RCTs; 119 participants). One trial found no difference between lubricating drops and no treatment in "end-of-day" comfort. The trial that compared saline with no treatment provided no results for the control group. Two studies measured corneal fluorescein staining on a scale of 0 to 20 (lower is better). We found low-certainty evidence of little to no difference between lubricating drops and no treatment in changes in the extent (MD -0.15 points, 95% CI -0.86 to 0.56; 2 RCTs; 119 participants), depth (MD -0.01 points, 95% CI -0.44 to 0.42; 2 RCTs; 119 participants), or type (MD 0.04 points, 95% CI -0.38 to 0.46; 2 RCTs; 119 participants) of corneal fluorescein staining scores. Regarding conjunctival redness, measured on a scale of 0 to 4 (lower is better), there was low-certainty evidence of little to no difference between lubricating drops and no treatment in nasal region scores (MD 0.10, 95% CI -0.29 to 0.49; 1 RCT; 73 participants) and temporal region scores (MD 0.00, 95% CI -0.39 to 0.39; 1 RCT; 73 participants). No studies reported microbial keratitis or inflammatory corneal infiltrates, and no trials reported vision-threatening adverse events up to four weeks of treatment. All trials reported the proportion of participants who discontinued participation. In two trials, no participants left any treatment group. Our meta-analysis of another two studies suggests little difference in the number of people who dropped out of the lubricating treatment group versus the no treatment group (risk ratio [RR] 1.42, 95% CI 0.19 to 10.94; 138 participants; low-certainty evidence). Lubricating drops versus saline Lubricating drops may have little to no effect compared with saline on contact lens discomfort measured on a visual analog scale of 0 to 100 (lower is better), but the evidence is very uncertain (MD 9.5 points, 95% CI -4.65 to 23.65; 1 RCT; 39 participants). No studies reported corneal fluorescein staining or conjunctival redness. No studies reported microbial keratitis or inflammatory corneal infiltrates, and no trials reported vision-threatening adverse events up to four weeks of treatment. Our meta-analysis of three studies suggests little difference in the number of people who dropped out of the lubricating treatment group versus the saline group (RR 1.56, 95% CI 0.47 to 5.12; 269 participants; low-certainty evidence).
Authors' conclusions: Very low-certainty evidence suggests that lubricating drops may improve contact lens discomfort compared with no treatment, but may have little or no effect on contact lens discomfort compared with saline. Low-certainty evidence also suggests that lubricating drops may have no unwanted effects that would lead to discontinuation over one to four weeks. Current evidence suggests that prescribing lubricating drops (including saline) to people with contact lens discomfort is a viable option. However, most studies did not assess patient-reported contact lens (dis)comfort using a validated instrument. Therefore, further well-designed trials are needed to generate high-certainty evidence on patient-reported outcomes as well as on longer-term safety outcomes.
Copyright © 2024 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
BC: none known. AP: Dr. Pucker has received research support from Alcon Research, LLC, Art Optical, ScienceBased Health, and the National Eye Institute. Dr. Pucker has served as a consultant for Alcon Research, LLC, Bausch and Lomb, Contamac, CooperVision, EpiTech, EyeGate Pharmaceuticals Inc., Euclid Systems, Hanall Biopharma, Kala Pharmaceuticals, Lexitas Pharma Services, Nevakar Inc., and Optikal Care Inc. Dr. Pucker is currently an employee of Lexitas Pharma Services. Lexitas Pharma Services did not have active artificial tear or rewetting drop studies during the conduct of this work. Dr. Pucker has received research and consulting fees related to articles included in this review. Dr. Pucker was not allowed to comment on these works, and he was not allowed to be the first or last author of this article to help mitigate his conflicts of interest. NC‐E: none known CJO: none known BH: Dr. Harkness has served as a consultant for Valley Contax, Inc. NC: Dr. Carnt reported receiving an Independent Medical Education Grant for Healthy Contact Lens Wear Australian Roadshow from Alcon Research, LLC; payment to institution. SL: reports a grant (UG1 EY020522) from the National Eye Institute, National Institutes of Health, USA; payment to institution. Dr. Liu is Managing Editor of Cochrane Review Group but was not involved in the editorial process for this review. AN: none known.
Figures
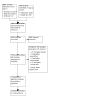








Update of
- doi: 10.1002/14651858.CD015751
References
References to studies included in this review
Barabino 2005 {published data only}
-
- Barabino S, Rolando M, Camicione P, Chen W, Calabria G. Effects of a 0.9% sodium chloride ophthalmic solution on the ocular surface of symptomatic contact lens wearers. Canadian Journal of Ophthalmology [Journal Canadien d'Ophtalmologie] 2005;40(1):45‐50. - PubMed
Keir 2006 {published data only}
-
- Dumbleton K, Jones L, Feng Y, Keir N, Woods CA, Simpson TL. The influence of a lubricant eye drop on contact lens discomfort in symptomatic hydrogel contact lens wearers. (unpublished manuscript).
-
- Feng Y, Dumbleton K, Keir N, Woods CA, Jones LW, Simpson TL, et al. The influence of a guar-based lubricant eye drop on ocular discomfort in symptomatic hydrogel contact lens wearers. Investigative Ophthalmology & Visual Science 2006;47:ARVO E‐abstract 2381.
-
- Keir NJ, Feng Y, Dumbleton K, Woods CA, Jones L, Simpson Tl, et al. The influence of a lubricant eye drop on ocular discomfort in symptomatic hydrogel contact lens wearers. American Academy of Optometry. Available at aaopt.org/past-meeting-abstract-archives 2006:Program No. 20129.
Malet 2003 {published data only}
-
- Malet F, Karsenti D, Pouliquen P. Preservative-free ocular hydrating agents in symptomatic contact lens wearers: saline versus PVP solution. Eye & Contact Lens 2003;29(1):38‐43. - PubMed
McDonald 2014 {published data only}
-
- McDonald M, Schachet JL, Lievens CW, Kern Jr. Systane® Ultra lubricant eye drops for treatment of contact lens-related dryness. Eye & Contact Lens 2014;40(2):106‐10. - PubMed
-
- NCT01132287. An evaluation of a marketed lubricated eye drop in soft contact lens wearers who experience ocular dryness. clinicaltrials.gov/show/NCT01132287 (first received 28 May 2010).
NCT02293538 {published data only}
-
- NCT02293538. Formula identification (FID) 114657 in contact lens wearers. clinicaltrials.gov/show/NCT02293538 (first received 5 August 2016).
Pucker 2020 {published data only}
-
- NCT03682809. Evaluation of systane complete for the treatment of contact lens discomfort. clinicalTrials.gov/show/NCT03682809 (first received by 6 April 2020).
-
- Pucker AD, McGwin G, Franklin QX, Nattis A, Lievens C. Evaluation of Systane® Complete for the treatment of contact lens discomfort. Contact Lens and Anterior Eye 2020;43(5):441‐7. - PubMed
Pucker 2021 {published data only}
-
- Brafford R, Lievens C, Pucker AD, Nattis A, McGwin G, Franklin Q, et al. Use of Systane® Complete over contact lenses to alleviate lens discomfort. Investigative Ophthalmology & Visual Science 2020;61(7):1487.
-
- NCT03848221. Direct application of systane complete to contact lenses. clinicaltrials.gov/show/NCT03848221 (first received 27 April 2020).
-
- Pucker AD, McGwin G, Franklin QX, Dubey J, Nattis A, Lievens C. Application of Systane® Complete for the treatment of contact lens discomfort. Contact Lens and Anterior eye 2021;44:101399. - PubMed
References to studies excluded from this review
ACTRN12619000274178 {published data only}
-
- ACTRN12619000274178. The effect of Systane® Complete and Systane® Hydration on comfort, vision and satisfaction with mini-scleral contact lens wear. trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12619000274178 (first received 25 February 2019).
ACTRN12619000773134 {published data only}
-
- ACTRN12619000773134. Influences of viscosity of lubricating eye drops on orthokeratology treatment outcomes. trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12619000773134 (first received 24 May 2019).
Asharlous 2016 {published data only}
Berry 1998 {published data only}
-
- Berry M, Pastis WK, Ellingham RB, Frost L, Corfield AP, Easty DL. Hyaluronan in dry eye and contact lens wearers. In: Sullivan DA, Dartt DA, Meneray MA, editor(s). Lacrimal Gland, Tear Film, and Dry Eye Syndromes 2. Advances in Experimental Medicine and Biology. Vol. 438. Boston: Springer, 1998:785-90. - PubMed
Caffery 1990 {published data only}
-
- Caffery BE, Josephson JE. Is there a better "comfort drop"? Journal of the American Optometric Association 1990;61(3):178‐82. - PubMed
Calvão‐Santos 2011 {published data only}
-
- Calvão-Santos G, Borges C, Nunes S, Salgado-Borges J, Duarte L. Efficacy of 3 different artificial tears for the treatment of dry eye in frequent computer users and/or contact lens users. European Journal of Ophthalmology 2011;21(5):538‐44. - PubMed
Carracedo 2018 {published data only}
-
- Carracedo G, Villa-Collar C, Martin-Gil A, Serramito M, Santamaría L. Comparison between viscous teardrops and saline solution to fill orthokeratology contact lenses before overnight wear. Eye & Contact Lens 2018;44 Suppl 1:S307‐11. - PubMed
ChiCTR2200066298 {published data only}
-
- ChiCTR2200066298. A prospective, multicenter, randomized, open, parallel positive controlled clinical study to evaluate the efficacy and safety of multi-functional nursing solution for rigid gas permeable contact lens wearers. trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2200066298 (first recieved 30 November 2022).
ChiCTR2300069668 {published data only}
-
- ChiCTR2300069668. Prospective, multicenter, randomized, parallel positive controlled clinical study to evaluate the efficacy and safety of rigid gas permeable contact lens lubrication for wearers of rigid breathable contact lenses. trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2300069668 (first received 23 March 2023).
CTRI/2022/07/043793 {published data only}
-
- CTRI/2022/07/043793. A post market clinical follow up prospective study to evaluate safety and performance of eye drops with (herbal extract) (sodium hyaluronate) with euphrasia extract eye drops. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2022/07/043793 (first received 6 July 2022).
CTRI/2022/07/043860 {published data only}
-
- CTRI/2022/07/043860. The purpose of the study is to evaluate safety and performance of Eye Preparations - Sodium hyaluronate with Euphrasia extract eye drops. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2022/07/043860 (first received 8 July 2022).
Downie 2018 {published data only}
-
- Downie LE, Gad A, Wong CY, Gray JH, Zeng W, Jackson DC, et al. Modulating contact lens discomfort with anti-inflammatory approaches: a randomized controlled trial. Investigative ophthalmology & visual science 2018;59(8):3755‐66. - PubMed
Dumbleton 2008 {published data only}
-
- Dumbleton K, Woods C, Schneider S, Fonn D. Comparison of signs and symptoms of dry eye when using ocular lubricants of differing viscosity. American Academy of Optometry. Available at aaopt.org/past-meeting-abstract-archives 2008:Program No. 21174.
Efron 1991 {published data only}
-
- Efron N, Golding TR, Brennan NA. The effect of soft lens lubricants on symptoms and lens dehydration. CLAO Journal 1991;17(2):114‐9. - PubMed
Gonzalez 2018 {published data only}
Guillon 2013 {published data only}
-
- Guillon M, Maissa C, Wong S, Lane A, Simmons P, Vehige J. Effect of lipid-based eyedrops on silicone hydrogel contact lens wear subjective acceptance. Contact Lens and Anterior Eye 2013;36:e43.
Guthrie 2015 {published data only}
-
- Guthrie SE, Jones LJ, Blackie CA, Korb DR. A comparative study between an oil-in-water emulsion and nonlipid eye drops used for rewetting contact lenses. Eye & Contact Lens 2015;41(6):373‐7. - PubMed
ISRCTN11194798 {published data only}
-
- ISRCTN11194798. Short-term evaluation of a commercial eyedrop in contact lens wearers. trialsearch.who.int/Trial2.aspx?TrialID=ISRCTN11194798 (first received 1 March 2019).
ISRCTN16230269 {published data only}
-
- ISRCTN16230269. Evaluation of two different eye drops in symptomatic soft contact lens wearers. trialsearch.who.int/Trial2.aspx?TrialID=ISRCTN16230269 (first received 7 June 2019).
Itoi 1995 {published data only}
-
- Itoi M, Kim O, Kimura T, Kanai A, Momose T, Kanki K, et al. Effect of sodium hyaluronate ophthalmic solution on peripheral staining of rigid contact lens wearers. CLAO Journal 1995;21(4):261‐4. - PubMed
Jeon 2021 {published data only}
-
- Jeon J, Park S. Comparison of the efficacy of eyelid warming masks and artificial tears for dry eye symptoms in contact lens wearers. Contact Lens & Anterior Eye 2021;44(1):30‐4. - PubMed
JPRN‐JRCT1031230239 {published data only}
-
- jRCT1031230239. Changes in ocular surface after instillation of eye drops containing PVP [Changes in tear film dynamics and ocular surface temperature after instillation of eye drops containing PVP in soft contact lens wearers]. jrct.niph.go.jp/latest-detail/jRCT1031230239 (first received 19 July 2023).
JPRN‐UMIN000010975 {published data only}
-
- JPRN-UMIN000010975. Effects of 3% diquafosol sodium ophthalmic solution on visual function in patient with dry eye wearing contact lens. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-UMIN000010975 (first received 30 June 2013).
JPRN‐UMIN000011397 {published data only}
-
- JPRN-UMIN000011397. A randomized study comparing topical rebamipide with artificial tears in the treatment of dry eye with contact lens wearers. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-UMIN000011397 (first received 7 August 2013).
JPRN‐UMIN000024064 {published data only}
-
- JPRN-UMIN000024064. Safety and efficacy of 3% diquafosol ophthalmic solution for soft contact lens-related dry eye. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-UMIN000024064 (first received 16 September 2016).
JPRN‐UMIN000031169 {published data only}
-
- JPRN-UMIN000031169. Evaluation of sense of commercial eye drops for contact lens wearers. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-UMIN000031169 (first received 7 February 2018).
Kading 2010 {published data only}
Liu 2020 {published data only}
-
- Liu L, Zhong X, Liu H, Chen Y. The influence of three lubricant eye drop on effects and ocular surface of myopia patients after orthokeratology lenses wearing. Zhonghua Shiyan Yanke Zazhi/Chinese Journal of Experimental Ophthalmology 2020;38(6):499-503.
NCT00469573 {published data only}
-
- NCT00469573. Safety and efficacy of optive in contact lens wearers with dry eyes. clinicaltrials.gov/ct2/show/NCT00469573 (first received 4 May 2007).
NCT00570843 {published data only}
-
- NCT00570843. Masked comparison of a new peg based artificial tear and optive for comfort, vision & wear time in patients wearing contact lenses. clinicaltrials.gov/show/NCT00570843 (first received 11 December 2007).
NCT00691197 {published data only}
-
- NCT00691197. Safety and acceptability of using a rewetting drop with contact lens wear. clinicaltrials.gov/show/NCT00691197 (first received 11 December 2007).
NCT00761202 {published data only}
-
- NCT00761202. Performance and acceptance of Optive® versus Hylocomod® eyedrops in patients with dry eye symptoms. clinicaltrials.gov/ct2/show/NCT00761202 (first received 29 September 2008).
NCT01051804 {published data only}
-
- NCT01051804. Evaluation of the repeated usage of Systane® Ultra eyedrop. clinicaltrials.gov/show/NCT01051804 (first received 20 January 2010).
NCT01105624 {published data only}
-
- NCT01105624. A four week study of azithromycin ophthalmic solution, 1% versus rewetting drops in subjects with history and current complaint of contact lens-related dry eye (CLDE). clinicaltrials.gov/ct2/show/NCT01105624 (first received 16 April 2010).
NCT01267656 {published data only}
-
- NCT01267656. Study to evaluate a contact lens lubricating and rewetting drop. clinicaltrials.gov/show/NCT01267656 (first received 28 December 2010).
NCT01384851 {published data only}
-
- NCT01384851. Efficacy of the chronic application of tear formulations. clinicaltrials.gov/ct2/show/NCT01384851 (first received 29 June 2011).
NCT01543061 {published data only}
-
- NCT01543061. Efficacy study of a new eyedrop on silicone hydrogel contact lens wearers with dry eye complaints. clinicaltrials.gov/ct2/show/NCT01543061 (first received 2 March 2012).
NCT01747616 {published data only}
-
- NCT01747616. Assessment of safety and tolerability of chitosan-n-acetylcysteine eye drops in subjects while wearing contact lenses and before insertion of contact lenses. clinicaltrials.gov/show/NCT01747616 (first received 11 December 2012).
NCT01844388 {published data only}
-
- NCT01844388. A study to compare a new eye drop formulation with Refresh Contacts®. clinicaltrials.gov/show/NCT01844388 (first received 1 May 2013).
NCT03050125 {published data only}
-
- NCT03050125. The impact of hypo-osmolar drops on contact lens comfort (SAFFRON). clinicaltrials.gov/ct2/show/NCT03050125 (first received 10 February 2017).
NCT03994406 {published data only}
-
- NCT03994406. Prolongation of contact lens comfortable wear duration by CLM2 Topical Gel. clinicaltrials.gov/show/NCT03994406 (first received 21 June 2019).
NCT04175340 {published data only}
-
- NCT04175340. A safety and effectiveness study of a new preservative free rewetting drop. classic.clinicaltrials.gov/show/NCT04175340 (first received 21 November 2019).
NCT04297618 {published data only}
-
- NCT04297618. The effect of Xiidra on comfort and dryness in symptomatic contact lens wearers. clinicaltrials.gov/ct2/show/NCT04297618 (first received 5 March 2020).
NCT04963543 {published data only}
-
- NCT04963543. Evaluation of comfort in symptomatic contact lens wearers. clinicaltrials.gov/ct2/show/NCT04963543 (first received 15 July 2021).
NCT05290727 {published data only}
-
- NCT05290727. Efficacy of "Aquoral Lipo" artificial tears in contact lens wearers with discomfort. clinicaltrials.gov/show/NCT05290727 (first received 22 March 2022).
NCT05505292 {published data only}
-
- NCT05505292. Lifitegrast 5% for the treatment of dry eye in habitual soft contact lens wearers. clinicaltrials.gov/ct2/show/NCT05505292 (first received 17 August 2022).
NCT05741216 {published data only}
-
- NCT05741216. Evaluation of comfort of ocular lubricants in symptomatic contact lens wearers. clinicaltrials.gov/show/NCT05741216 (first received 23 February 2023).
NCT05814367 {published data only}
-
- NCT05814367. Clinical investigation of visual acuity in contact lens wearers after instillation of investigational lubricating eye drops. clinicaltrials.gov/show/NCT05814367 (first received 3 April 2023).
NCT05902364 {published data only}
-
- NCT05902364. Systane® Ultra preservative free lubricant eye drops. classic.clinicaltrials.gov/show/NCT05902364 (first received 5 June 2023).
NCT05932225 {published data only}
-
- NCT05932225. Systane® Complete preservative free lubricant eye drops. classic.clinicaltrials.gov/show/NCT05932225 (first received 27 June 2023).
NCT06131476 {published data only}
-
- NCT06131476. Clinical investigation of visual acuity in contact lens wearers after instillation of a lipid-based lubricating eye drop. classic.clinicaltrials.gov/show/NCT06131476 (first received 9 November 2023).
Nichols 2016 {published data only}
-
- Simmons P, Liu H, Chen R, Math FL, Carlisle C, Vehige J. Clinical evaluation of a dual-polymer rewetting drop: improved patient-reported outcomes and lid wiper epitheliopathy in contact lens wearers. Contact Lens & Anterior Eye 2015;38:e33.
Ogami 2021 {published data only}
-
- Ogami T, Asano H, Hiraoka T, Yamada Y, Oshika T. The effect of diquafosol ophthalmic solution on clinical parameters and visual function in soft contact lens-related dry eye. Advanced Therapy 2021;38(11):5534-47. - PubMed
Ozkan 2004 {published data only}
-
- Ozkan JJ, Snoxall B, Maher A, Papas E. Lubricants and their effect on comfort with silicone hydrogel and conventional hydrogel lens wear. Investigative Ophthalmology & Visual Science 2004;45:ARVO E‐abstract 1551.
Ozkan 2005 {published data only}
-
- Ozkan J, Papas E. Effect of dry eye lubricant drops on symptomatic silicone hydrogel wearers. American Academy of Optometry. Available at aaopt.org/past-meeting-abstract-archives 2005:Program No. 19162.
Rohit 2016 {published data only}
-
- Rohit A, Willcox MD, Stapleton F. Effects of lipid supplements on tear biochemistry in contact lens wearers. Optometry and Vision Science 2016;93(10):1203-9. - PubMed
Rohit 2017 {published data only}
-
- Rohit A, Willcox Md, Stapleton F. Lipid supplements and clinical aspects of tear film in habitual lens wearers. Optometry and Vision Science 2017;94(2):174‐82. - PubMed
Sasai 2009 {published data only}
-
- Sasai A, Vehige J, Simmons P, Beard B, Lai F. Clinical evaluation of an artificial tear in contact lens wearers. American Academy of Optometry. Available at aaopt.org/past-meeting-abstract-archives 2009:Program No. 21747.
Tavazzi 2020 {published data only}
Xu 2023 {published data only}
-
- Xu Y, Chen S, Wu D, Yuan Z, Liu X. Effect of sodium hyaluronate eye drop on tear film stability and tear secretion in patients with rigid gas-permeable contact lens-associated xerophthalmia. Tropical Journal of Pharmaceutical Research 2023;22(12):2545‐51. [DOI: 10.4314/tjpr.v22i12.18] - DOI
References to studies awaiting assessment
NCT02956083 {published data only}
-
- NCT02956083. The effect of tear supplements on contact lens comfort. clinicaltrials.gov/show/NCT02956083 (first received 4 November 2016).
Additional references
Baudouin 2010
-
- Baudouin C, Labbé A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Progress in Retinal and Eye Research 2010;29(4):312-34. [PMID: ] - PubMed
Begley 2001
-
- Begley CG, Chalmers RL, Mitchell G, Nichols KK, Caffery B, Simpson T, et al. Characterization of ocular surface symptoms from optometric practices in North America. Cornea 2001;20(6):610-8. [PMID: ] - PubMed
Brennan 1989
-
- Brennan NA, Efron N. Symptomatology of HEMA contact lens wear. Optometry and Vision Science 1989;66:834-8. - PubMed
Chalmers 2006
-
- Chalmers RL, Begley CG. Dryness symptoms among an unselected clinical population with and without contact lens wear. Contact Lens and Anterior Eye 2006;29(1):25-30. [PMID: ] - PubMed
Chalmers 2012
-
- Chalmers RL, Begley CG, Moody K, Hickson-Curran SB. Contact Lens Dry Eye Questionnaire-8 (CLDEQ-8) and opinion of contact lens performance. Optometry and Vision Science 2012;89(10):1435-42. [PMID: ] - PubMed
Chalmers 2016
-
- Chalmers RL, Keay L, Hickson-Curran SB, Gleason WJ. Cutoff score and responsiveness of the 8-item Contact Lens Dry Eye Questionnaire (CLDEQ-8) in a large daily disposable contact lens registry. Contact Lens and Anterior Eye 2016;39(5):342-52. [PMID: ] - PubMed
Cohen 1988
-
- Cohen J. Statistical power analysis in the behavioral sciences. 2nd edition. Hillsdale (NJ): Lawrence Erlbaum Associates Inc, 1988.
Covidence [Computer program]
-
- Covidence. Melbourne, Australia: Veritas Health Innovation, Accessed 25 October 2022. Available at covidence.org.
Craig 2013
-
- Craig JP, Willcox MD, Argueso P, Maissa C, Stahl U, Tomlinson A, members of TFOS International Workshop on Contact Lens Discomfort. The TFOS International Workshop on Contact Lens Discomfort: report of the contact lens interactions with the tear film subcommittee. Investigative Ophthalmology and Visual Science 2013;54(11):TFOS123-56. [PMID: ] - PubMed
Craig 2017
-
- Craig JP, Nelson JD, Azar DT, Belmonte C, Bron AJ, Chauhan SK, et al. TFOS DEWS II Report Executive Summary. Ocular Surface 2017;15(4):802-12. [PMID: ] - PubMed
Deeks 2022
-
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook/archive/v6.3.
Dogru 2011
-
- Dogru M, Tsubota K. Pharmacotherapy of dry eye. Expert Opinion on Pharmacotherapy 2011;12(3):325-34. [PMID: ] - PubMed
Doughty 1997
-
- Doughty MJ, Fonn D, Richter D, Simpson T, Caffery B, Gordon K. A patient questionnaire approach to estimating the prevalence of dry eye symptoms in patients presenting to optometric practices across Canada. Optometry and Vision Science 1997;74(8):624-31. [PMID: ] - PubMed
Dumbleton 2013
-
- Dumbleton K, Caffery B, Dogru M, Hickson-Curran S, Kern J, Kojima T, members of the TFOS International Workshop on Contact Lens Discomfort. The TFOS International Workshop on Contact Lens Discomfort: report of the subcommittee on epidemiology. Investigative Ophthalmology and Visual Science 2013;54(11):TFOS20-36. [PMID: ] - PubMed
Efron 2013
-
- Efron N, Jones L, Bron AJ, Knop E, Arita R, Barabino S, members of the TFOS International Workshop on Contact Lens Discomfort. The TFOS International Workshop on Contact Lens Discomfort: report of the contact lens interactions with the ocular surface and adnexa subcommittee. Investigative Ophthalmology and Visual Science 2013;54(11):TFOS98-122. [PMID: ] - PubMed
Efron 2016
-
- Efron N, Brennan NA, Morgan PB, Wilson T. Lid wiper epitheliopathy. Progress in Retinal and Eye Research 2016;53:140-74. [PMID: ] - PubMed
Egger 1997
Fernandez‐Jimenez 2022
-
- Fernández-Jimenez E, Diz-Arias E, Peral A. Improving ocular surface comfort in contact lens wearers. Contact Lens and Anterior Eye 2022;45(3):101544. [PMID: ] - PubMed
Gad 2019
-
- Gad A, Vingrys AJ, Wong CY, Jackson DC, Downie LE. Tear film inflammatory cytokine upregulation in contact lens discomfort. Ocular Surface 2019;17(1):89-97. [PMID: ] - PubMed
Glanville 2006
Higgins 2022
-
- Higgins JP, Li T, Deeks JJ, editor(s). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook/archive/v6.3.
Higgins 2023a
-
- Higgins JP, Eldridge S, Li T. Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook.
Higgins 2023b
-
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (updated August 2023). Cochrane, 2023. Available from training.cochrane.org/handbook.
Hu 2022
Jones 2023
-
- Jones L, Efron N, Bandamwar K, Barnett M, Jacobs DS, Jalbert I, et al. TFOS Lifestyle: impact of contact lenses on the ocular surface. Ocular Surface 2023;29:175-219. - PubMed
Koh 2020
Nair 2022
Nichols 2006
-
- Nichols JJ, Sinnott LT. Tear film, contact lens, and patient-related factors associated with contact lens-related dry eye. Investigative Ophthalmology and Visual Science 2006;47(4):1319-28. [PMID: ] - PubMed
Nichols 2013
-
- Nichols JJ, Willcox MD, Bron AJ, Belmonte C, Ciolino JB, Craig JP, members of the TFOS International Workshop on Contact Lens Discomfort. The TFOS International Workshop on Contact Lens Discomfort: executive summary. Investigative Ophthalmology and Visual Science 2013;54(11):TFOS7–13. [PMID: ] - PMC - PubMed
Page 2021
Page 2023
-
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (updated August 2023). Cochrane, 2023. Available from training.cochrane.org/handbook.
Papas 2011
-
- Papas EB, Keay L, Golebiowski B. Estimating a just-noticeable difference for ocular comfort in contact lens wearers. Investigative Ophthalmology and Visual Science 2011;52(7):4390-4. [PMID: ] - PubMed
Papas 2013
-
- Papas EB, Ciolino JB, Jacobs D, Miller WL, Pult H, Sahin A, members of the TFOS International Workshop on Contact Lens Discomfort. The TFOS International Workshop on Contact Lens Discomfort: report of the management and therapy subcommittee. Investigative Ophthalmology and Visual Science 2013;54(11):TFOS183-203. [PMID: ] - PubMed
Peacock 2008
Pucker 2020a
Pucker 2020b
-
- Pucker AD. A review of the compatibility of topical artificial tears and rewetting drops with contact lenses. Contact Lens and Anterior Eye 2020;43(5):426-32. [PMID: ] - PubMed
RevMan 2023 [Computer program]
-
- Review Manager (RevMan). Version 4.24.0. The Cochrane Collaboration, 2023. Available at revman.cochrane.org.
Rubinstein 1997
-
- Rubinstein MP, Evans JE. Therapeutic contact lenses and eyedrops — is there a problem? Contact Lens and Anterior Eye 1997;20(1):9-11. - PubMed
Rueff 2021
-
- Rueff EM, Tichenor AA, Ngo W, Pucker AD. A review of meibomian gland structure, function, and contact lens wear. Contact Lens and Anterior Eye 2021;45(5):101560. [PMID: ] - PubMed
Saldanha 2018
Schünemann 2023
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH. Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (updated August 2023). Cochrane, 2023. Available from training.cochrane.org/handbook.
Stahl 2018
-
- Stahl U, Jalbert I. Exploring the links between contact lens comfort, osmolarity and lid wiper staining. Contact Lens and Anterior Eye 2018;41(1):110-6. [PMID: ] - PubMed
Stapleton 2017
-
- Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, et al. TFOS DEWS II Epidemiology Report. Ocular Surface 2017;15(3):334-65. - PubMed
Stapleton 2021
-
- Stapleton F, Bakkar M, Carnt N, Chalmers R, Vijay AK, Marasini S, et al. CLEAR - Contact lens complications. Contact Lens and Anterior Eye 2021;44(2):330-67. [PMID: ] - PubMed
Sterne 2019
-
- Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [PMID: ] - PubMed
Uchino 2008a
-
- Uchino M, Schaumberg DA, Dogru M, Uchino Y, Fukagawa K, Shimmura S, et al. Prevalence of dry eye disease among Japanese visual display terminal users. Ophthalmology 2008;115(11):1982-8. [PMID: ] - PubMed
Uchino 2008b
-
- Uchino M, Dogru M, Uchino Y, Fukagawa K, Shimmura S, Takebayashi T, et al. Japan Ministry of Health Study on prevalence of dry eye disease among Japanese high school students. American Journal of Ophthalmology 2008;146(6):925-9.e2. [PMID: ] - PubMed
USFDA 510k Guidance
-
- US Food and Drug Administration. Contact Lens Care Products – Premarket Notification 510(k) Guidance. www.fda.gov/medical-devices/guidance-documents-medical-devices-and-radia... 1997 (Content current as of 03/22/2018; Accessed 24/04/2023).
USFDA CFR Part 349
-
- Food and Drug Administration (FDA), Department of Health and Human Services (DHHS). Part 349 - Ophthalmic drug products for over-the-counter human use. www.govinfo.gov/content/pkg/CFR-2021-title21-vol5/pdf/CFR-2021-title21-v... 2021 (Content current as of 28 March 2023; Accessed 24 April 2023).
Vajdic 1999
-
- Vajdic C, Holden BA, Sweeney DF, Cornish RM. The frequency of ocular symptoms during spectacle and daily soft and rigid contact lens wear. Optometry and Vision Science 1999;76(10):705-11. [PMID: ] - PubMed
Walsh 2019
WebPlotDigitizer [Computer program]
-
- WebPlotDigitizer. Version 4.6. Rohatgi A, September 2022. Available at automeris.io/WebPlotDigitizer.
Young 2002
-
- Young G, Veys J, Pritchard N, Coleman S. A multi-centre study of lapsed contact lens wearers. Ophthalmic and Physiological Optics 2002;22(6):516-27. [PMID: ] - PubMed
Young 2011
-
- Young G, Chalmers RL, Napier L, Hunt C, Kern J. Characterizing contact lens-related dryness symptoms in a cross-section of UK soft lens wearers. Contact Lens and Anterior Eye 2011;34(2):64-70. [PMID: ] - PubMed
Zhang 2012
-
- Zhang Y, Chen H, Wu X. Prevalence and risk factors associated with dry eye syndrome among senior high school students in a county of Shandong province, China. Ophthalmic Epidemiology 2012;19(4):226-30. [PMID: ] - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

