Scaled Complexity of Mammalian Astrocytes: Insights From Mouse and Macaque
- PMID: 39235147
- PMCID: PMC11378921
- DOI: 10.1002/cne.25665
Scaled Complexity of Mammalian Astrocytes: Insights From Mouse and Macaque
Abstract
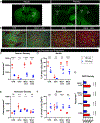
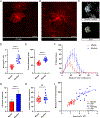
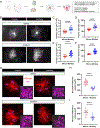
Astrocytes intricately weave within the neuropil, giving rise to characteristic bushy morphologies. Pioneering studies suggested that primate astrocytes are more complex due to increased branch numbers and territory size compared to rodent counterparts. However, there has been no comprehensive comparison of astrocyte morphology across species. We employed several techniques to investigate astrocyte morphology and directly compared them between mice and rhesus macaques in cortical and subcortical regions. We assessed astrocyte density, territory size, branching structure, fine morphological complexity, and interactions with neuronal synapses using a combination of techniques, including immunohistochemistry, adeno-associated virus-mediated transduction of astrocytes, diOlistics, confocal imaging, and electron microscopy. We found significant morphological similarities between primate and rodent astrocytes, suggesting that astrocyte structure has scaled with evolution. Our findings show that primate astrocytes are larger and more numerous than those in rodents but contest the view that primate astrocytes are morphologically far more complex.
Keywords: astrocyte; astrocyte complexity; astrocyte morphology; astrocyte‐synapse interaction; species comparison.
© 2024 Wiley Periodicals LLC.
Figures



References
-
- Aten S, Kiyoshi CM, Arzola EP, Patterson JA, Taylor AT, Du Y, Guiher AM, Philip M, Camacho EG, Mediratta D, Collins K, Boni K, Garcia SA, Kumar R, Drake AN, Hegazi A, Trank L, Benson E, Kidd G, Terman D, Zhou M, 2022. Ultrastructural view of astrocyte arborization, astrocyte-astrocyte and astrocyte-synapse contacts, intracellular vesicle-like structures, and mitochondrial network. Prog. Neurobiol 213, 102264. 10.1016/j.pneurobio.2022.102264 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

