IMT-P8 potentiates Gram-positive specific antibiotics in intrinsically resistant Gram-negative bacteria
- PMID: 39235250
- PMCID: PMC11459922
- DOI: 10.1128/aac.00753-24
IMT-P8 potentiates Gram-positive specific antibiotics in intrinsically resistant Gram-negative bacteria
Abstract
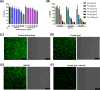
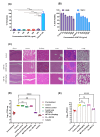
Gram-negative bacteria (GNB) pose a major global public health challenge as they exhibit a remarkable level of resistance to antibiotics. One of the factors responsible for promoting resistance against a wide range of antibiotics is the outer membrane (OM) of Gram-negative bacteria. The OM acts as a barrier that prevents the entry of numerous antibiotics by reducing their influx (due to membrane impermeability) and enhancing their efflux (with the help of efflux pumps). Our study focuses on analyzing the effect of IMT-P8, a cell-penetrating peptide (CPP), to enhance the influx of various Gram-positive specific antibiotics in multi-drug resistant Gram-negative pathogens. In the mechanistic experiments, IMT-P8 permeabilizes the OM at the same concentrations at which it enhances the activity of various antibiotics against GNB. Cytoplasmic membrane permeabilization was also observed at these concentrations, indicating that IMT-P8 acts on both the outer and cytoplasmic membranes. IMT-P8 interferes with the intrinsic resistance mechanism of GNB and has the potential to make Gram-positive specific antibiotics effective against GNB. IMT-P8 extends the post-antibiotic effect and in combination with antibiotics shows anti-persister activity. The IMT-P8/fusidic acid combination is effective in eliminating intracellular pathogens. IMT-P8 with negligible toxicity displayed good efficacy in murine lung and thigh infection models. Based on these findings, IMT-P8 is a potential antibiotic adjuvant to treat Gram-negative bacterial infections that pose a health hazard.
Keywords: A. baumannii; Gram-negative bacteria; antibiotic potentiation; cell-penetrating peptide; combination therapy; membrane permeabilization; multi-drug resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

