The three Plasmodium falciparum Aurora-related kinases display distinct temporal and spatial associations with mitotic structures in asexual blood stage parasites and gametocytes
- PMID: 39235260
- PMCID: PMC11423587
- DOI: 10.1128/msphere.00465-24
The three Plasmodium falciparum Aurora-related kinases display distinct temporal and spatial associations with mitotic structures in asexual blood stage parasites and gametocytes
Abstract
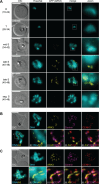
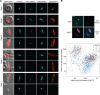
Aurora kinases are crucial regulators of mitotic cell cycle progression in eukaryotes. The protozoan malaria parasite Plasmodium falciparum replicates via schizogony, a specialized mode of cell division characterized by consecutive asynchronous rounds of nuclear division by closed mitosis followed by a single cytokinesis event producing dozens of daughter cells. P. falciparum encodes three Aurora-related kinases (PfARKs) that have been reported essential for parasite proliferation, but their roles in regulating schizogony have not yet been explored in great detail. Here, we engineered transgenic parasite lines expressing GFP-tagged PfARK1-3 to provide a systematic analysis of their expression timing and subcellular localization throughout schizogony as well as in the non-dividing gametocyte stages, which are essential for malaria transmission. We demonstrate that all three PfARKs display distinct and highly specific and exclusive spatiotemporal associations with the mitotic machinery. In gametocytes, PfARK3 is undetectable, and PfARK1 and PfARK2 show male-specific expression in late-stage gametocytes, consistent with their requirement for endomitosis during male gametogenesis in the mosquito vector. Our combined data suggest that PfARK1 and PfARK2 have non-overlapping roles in centriolar plaque maturation, assembly of the mitotic spindle, kinetochore-spindle attachment and chromosome segregation, while PfARK3 seems to be exquisitely involved in daughter cell cytoskeleton assembly and cytokinesis. These important new insights provide a reliable foundation for future research aiming at the functional investigation of these divergent and possibly drug-targetable Aurora-related kinases in mitotic cell division of P. falciparum and related apicomplexan parasites.IMPORTANCEMalaria parasites replicate via non-conventional modes of mitotic cell division, such as schizogony, employed by the disease-causing stages in the human blood or endomitosis during male gametogenesis in the mosquito vector. Understanding the molecular mechanisms regulating cell division in these divergent unicellular eukaryotes is not only of scientific interest but also relevant to identify potential new antimalarial drug targets. Here, we carefully examined the subcellular localization of all three Plasmodium falciparum Aurora-related kinases (ARKs), distantly related homologs of Aurora kinases that coordinate mitosis in model eukaryotes. Detailed fluorescence microscopy-based analyses revealed distinct, specific, and exclusive spatial associations for each parasite ARK with different components of the mitotic machinery and at different phases of the cell cycle during schizogony and gametocytogenesis. This comprehensive set of results closes important gaps in our fragmentary knowledge on this important group of kinases and offers a valuable source of information for future functional studies.
Keywords: Aurora-related kinase; Plasmodium falciparum; cell division; gametocytes; malaria; mitosis; schizogony.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Kafsack BFC, Rovira-Graells N, Clark TG, Bancells C, Crowley VM, Campino SG, Williams AE, Drought LG, Kwiatkowski DP, Baker DA, Cortés A, Llinás M. 2014. A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature New Biol 507:248–252. doi: 10.1038/nature12920 - DOI - PMC - PubMed
-
- Sinha A, Hughes KR, Modrzynska KK, Otto TD, Pfander C, Dickens NJ, Religa AA, Bushell E, Graham AL, Cameron R, Kafsack BFC, Williams AE, Llinas M, Berriman M, Billker O, Waters AP. 2014. A cascade of DNA-binding proteins for sexual commitment and development in Plasmodium. Nature New Biol 507:253–257. doi: 10.1038/nature12970 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

