Feedstock variability impacts the bioconversion of sugar and lignin streams derived from corn stover by Clostridium tyrobutyricum and engineered Pseudomonas putida
- PMID: 39235453
- PMCID: PMC11376215
- DOI: 10.1111/1751-7915.70006
Feedstock variability impacts the bioconversion of sugar and lignin streams derived from corn stover by Clostridium tyrobutyricum and engineered Pseudomonas putida
Abstract
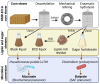
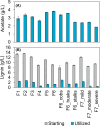
Feedstock variability represents a challenge in lignocellulosic biorefineries, as it can influence both lignocellulose deconstruction and microbial conversion processes for biofuels and biochemicals production. The impact of feedstock variability on microbial performance remains underexplored, and predictive tools for microbial behaviour are needed to mitigate risks in biorefinery scale-up. Here, twelve batches of corn stover were deconstructed via deacetylation, mechanical refining, and enzymatic hydrolysis to generate lignin-rich and sugar streams. These batches and their derived streams were characterised to identify their chemical components, and the streams were used as substrates for producing muconate and butyrate by engineered Pseudomonas putida and wildtype Clostridium tyrobutyricum, respectively. Bacterial performance (growth, product titers, yields, and productivities) differed among the batches, but no strong correlations were identified between feedstock composition and performance. To provide metabolic insights into the origin of these differences, we evaluated the effect of twenty-three isolated chemical components on these microbes, including three components in relevant bioprocess settings in bioreactors, and we found that growth-inhibitory concentrations were outside the ranges observed in the streams. Overall, this study generates a foundational dataset on P. putida and C. tyrobutyricum performance to enable future predictive models and underscores their resilience in effectively converting fluctuating lignocellulose-derived streams into bioproducts.
© 2024 The Author(s). Microbial Biotechnology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Almqvist, H. , Veras, H. , Li, K. , Garcia Hidalgo, J. , Hulteberg, C. , Gorwa‐Grauslund, M. et al. (2021) Muconic acid production using engineered Pseudomonas putida KT2440 and a guaiacol‐rich fraction derived from Kraft lignin. ACS Sustainable Chemistry & Engineering, 9, 8097–8106.
-
- Alriksson, B. , Cavka, A. & Jönsson, L.J. (2011) Improving the fermentability of enzymatic hydrolysates of lignocellulose through chemical in‐situ detoxification with reducing agents. Bioresource Technology, 102, 1254–1263. - PubMed
-
- Alt, H.M. , Benson, A.F. , Haugen, S.J. , Ingraham, M.A. , Michener, W.E. , Woodworth, S.P. et al. (2024) Analysis of sugars, small organic acids, and alcohols by HPLC‐RID V.2 Protocols.io .
-
- Banerjee, N. , Bhatnagar, R. & Viswanathan, L. (1981) Inhibition of glycolysis by furfural in Saccharomyces cerevisiae . European Journal of Applied Microbiology and Biotechnology, 11, 226–228.
-
- Berni Canani, R. , Terrin, G. , Cirillo, P. , Castaldo, G. , Salvatore, F. , Cardillo, G. et al. (2004) Butyrate as an effective treatment of congenital chloride diarrhea. Gastroenterology, 127, 630–634. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

