SAFFRON-104: a phase Ib/II study of sitravatinib alone or with tislelizumab in advanced hepatocellular carcinoma and gastric cancer/gastroesophageal junction cancer
- PMID: 39235596
- PMCID: PMC11377389
- DOI: 10.1007/s00262-024-03806-2
SAFFRON-104: a phase Ib/II study of sitravatinib alone or with tislelizumab in advanced hepatocellular carcinoma and gastric cancer/gastroesophageal junction cancer
Abstract
Background: Sitravatinib is a spectrum-selective tyrosine kinase inhibitor targeting TAM (TYRO3, AXL, MER), VEGFR-2, KIT, and MET. SAFFRON-104 (NCT03941873) was a multicohort phase Ib/II study investigating sitravatinib with/without tislelizumab, an anti-programmed cell death protein 1 (PD-1) antibody, in patients with advanced hepatocellular carcinoma (HCC) or gastric cancer/gastroesophageal junction cancer (GC/GEJC).
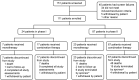
Methods: Eligible patients had histologically/cytologically confirmed advanced HCC or GC/GEJC. Phase I determined the recommended phase II dose (RP2D) of sitravatinib with/without tislelizumab. Phase II evaluated sitravatinib monotherapy in patients with pretreated HCC, and sitravatinib plus tislelizumab in anti-PD-(L)1-naïve or -treated HCC and anti-PD-(L)1-naïve GC/GEJC. Primary endpoints were safety/tolerability (phase I) and objective response rate (ORR) (phase II).
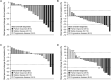
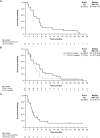
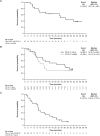
Results: At data cutoff (March 31, 2023), 111 patients were enrolled; 102 were efficacy-evaluable (median study follow-up 9.1 months [range: 0.7-36.9]). The RP2D of sitravatinib was determined as 120 mg orally once daily. In patients receiving sitravatinib monotherapy and sitravatinib in combination with tislelizumab, grade ≥ 3 treatment-related adverse events occurred in 14 (51.9%) and 42 (50.0%) patients, respectively. The ORR was 25% (95% confidence interval [CI]: 8.7-49.1) in patients with pretreated HCC receiving sitravatinib monotherapy. In patients receiving sitravatinib with tislelizumab, the ORR was 11.5% (95% CI 2.4-30.2) with anti-PD-(L)1-naïve HCC, 9.5% (95% CI 1.2-30.4) with anti-PD-(L)1-treated HCC, and 16.1% (95% CI 5.5-33.7) in patients with anti-PD-(L)1-naïve GC/GEJC.
Conclusions: Sitravatinib with/without tislelizumab was generally well tolerated and showed preliminary antitumor activity in patients with advanced HCC and GC/GEJC.
Keywords: Clinical trials; Combination therapy; Drug therapy; Immune checkpoint inhibitors; Immunotherapy; Phase II.
© 2024. The Author(s).
Conflict of interest statement
Jin Li reports payments or honoraria for consulting or speakers bureaus from ArteMed Hospital, Novartis, HengRui, BI, and Lilly; Jieer Ying and Hongming Pan report stocks or shares in BeiGene (institutional relationship with financial interest); Zhenggang Ren reports a consulting or advisory role with AstraZeneca, Roche, and Merck Sharp & Dohme; Zhang Zhang and Juan Zhang report financial relationships with BeiGene; Xin Li and Fan Yu report full-time employment by BeiGene; Yuxian Bai, Zhendong Chen, Yabing Guo, Weijia Fang, Feng Zhang, Jianping Xiong, Tao Zhang, Zhiqiang Meng, Jingdong Zhang, Chunyi Hao, Yajin Chen, Xiaoyan Lin, Fuxiang Zhou, and Shukui Qin have no potential conflict of interest to report.
Figures
References
-
- Rumgay H, Ferlay J, de Martel C, Georges D, Ibrahim AS, Zheng R, Wei W, Lemmens V, Soerjomataram I (2022) Global, regional and national burden of primary liver cancer by subtype. Eur J Cancer 161:108–118. 10.1016/j.ejca.2021.11.023 - PubMed
-
- Ajani JA, Lee J, Sano T, Janjigian YY, Fan D, Song S (2017) Gastric adenocarcinoma. Nat Rev Dis Primers 3:17036. 10.1038/nrdp.2017.36 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

