Active template synthesis
- PMID: 39235620
- PMCID: PMC11376342
- DOI: 10.1039/d4cs00430b
Active template synthesis
Abstract
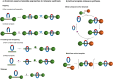
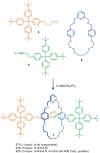
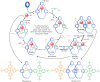
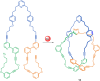
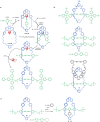
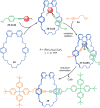
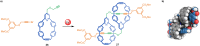
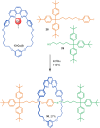
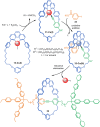
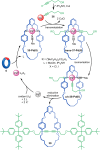
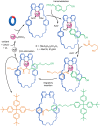
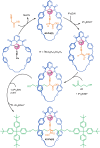
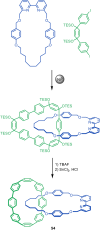
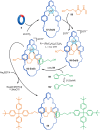
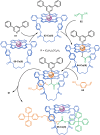
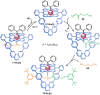
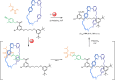
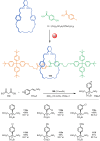
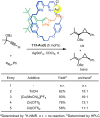
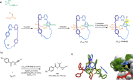
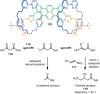
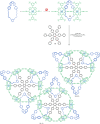
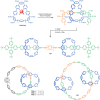
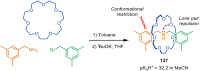
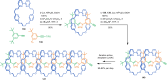
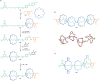
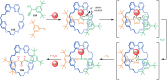
The active template synthesis of mechanically interlocked molecular architectures exploits the dual ability of various structural elements (metals or, in the case of metal-free active template synthesis, particular arrangements of functional groups) to serve as both a template for the organisation of building blocks and as a catalyst to facilitate the formation of covalent bonds between them. This enables the entwined or threaded intermediate structure to be covalently captured under kinetic control. Unlike classical passive template synthesis, the intercomponent interactions transiently used to promote the assembly typically do not 'live on' in the interlocked product, meaning that active template synthesis can be traceless and used for constructing mechanically interlocked molecules that do not feature strong binding interactions between the components. Since its introduction in 2006, active template synthesis has been used to prepare a variety of rotaxanes, catenanes and knots. Amongst the metal-ion-mediated versions of the strategy, the copper(I)-catalysed alkyne-azide cycloaddition (CuAAC) remains the most extensively used transformation, although a broad range of other catalytic reactions and transition metals also provide effective manifolds. In metal-free active template synthesis, the recent discovery of the acceleration of the reaction of primary amines with electrophiles through the cavity of crown ethers has proved effective for forming an array of rotaxanes without recognition elements, including compact rotaxane superbases, dissipatively assembled rotaxanes and molecular pumps. This Review details the active template concept, outlines its advantages and limitations for the synthesis of interlocked molecules, and charts the diverse set of reactions that have been used with this strategy to date. The application of active template synthesis in various domains is discussed, including molecular machinery, mechanical chirality, catalysis, molecular recognition and various aspects of materials science.
Conflict of interest statement
There are no conflicts of interests to declare.
Figures




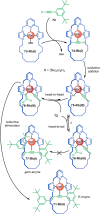

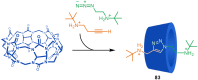

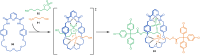
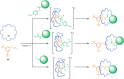














References
-
- Frisch H. Martin I. Mark H. Monatsh. Chem. 1953;84:250–256. doi: 10.1007/BF00899187. - DOI
-
- Frisch H. L. Wasserman E. J. Am. Chem. Soc. 1961;83:3789–3795. doi: 10.1021/ja01479a015. - DOI
-
- Wasserman E. J. Am. Chem. Soc. 1960;82:4433–4434. doi: 10.1021/ja01501a082. - DOI
-
- Harrison I. T. Harrison S. J. Am. Chem. Soc. 1967;89:5723–5724. doi: 10.1021/ja00998a052. - DOI
Publication types
LinkOut - more resources
Full Text Sources

