Fludrocortisone dose-response relationship in septic shock: a randomised phase II trial
- PMID: 39235623
- PMCID: PMC11588801
- DOI: 10.1007/s00134-024-07616-z
Fludrocortisone dose-response relationship in septic shock: a randomised phase II trial
Abstract
Background: The combination of intravenous hydrocortisone and enteral fludrocortisone may reduce mortality in patients with septic shock. The optimal dose and reliability of absorption of fludrocortisone in critically ill patients are unclear.
Methods: In a multi-centre, open label, phase II randomized clinical trial, intravenous hydrocortisone alone or in combination with one of three doses of enteral fludrocortisone (50 µg, 100 µg or 200 µg daily) for 7 days was compared in patients with septic shock. The primary outcome was time to shock resolution. We conducted pharmacokinetic studies to assess absorption.
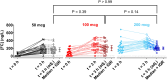
Results: Out of 153 enrolled patients, 38 (25%) received hydrocortisone alone, 42 (27%) received additional 50 µg, 36 (24%) received 100 µg and 37 (24%) received 200 µg fludrocortisone. Plasma concentrations of fludrocortisone were detected in 97% of patients at 3 h-median (interquartile range [IQR]) 261 (156-334) ng/L. There was no significant difference in the time to shock resolution between groups with median (IQR) of 3 (2.5-4.5), 3 (2-4), 3 (2-6) and 3 (2-5.5) days in the hydrocortisone alone, 50 µg, 100 µg and 200 µg fludrocortisone groups, respectively. The corresponding 28-day mortality rates were 9/38 (24%), 7/42 (17%), 4/36 (11%) and 4/37 (11%), respectively. There were no significant differences between groups with respect to, recurrence of shock, indices of organ failure or other secondary outcomes.
Conclusions: Enteral fludrocortisone resulted in detectable plasma fludrocortisone concentrations in the majority of critically ill patients with septic shock, although they varied widely indicating differing absorption and bioavailability. Its addition to hydrocortisone was not associated with shorter time to shock resolution.
Keywords: Fludrocortisone; Hydrocortisone; Pharmacokinetics; Septic shock.
© 2024. Crown.
Conflict of interest statement
Declarations. Conflicts of interest: BV, JM, NH and SF are supported by a Leadership Fellowship and AB from an Ideas Grant from the National Health and Medical Research Council of Australia. BV and NH receive institutional research support from Baxter for the conduct of a clinical trial in patients with diabetic ketoacidosis. AB is supported by an Australian Research Council Future Fellowship and grants from the Translational Research Institute, Australian Infectious diseases Research Centre and Metro South health. JD, MR and LM are supported by a grant from the Hospital Research Foundation. MY is supported by a grant from the Baker Foundation and the Australian Research Council. SF has received consulting fees from RevImmune Inc and is Director of Sepsis Australia (honorary) and Asia Pacific Sepsis Alliance (honorary). All other others do not report any conflict of interest.
Figures
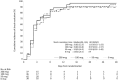
 ), 100 (
), 100 ( ) and 200 (
) and 200 ( ) mcg. Also shown are the 3 h values for each dosing regimen after adjusting for elimination of their pre-dose over the 3 h dosing interval (
) mcg. Also shown are the 3 h values for each dosing regimen after adjusting for elimination of their pre-dose over the 3 h dosing interval ( ), along with the median ± IQR for each group. No significant difference between the adjusted values was found with a Kruskal–Wallis test (p > 0.05)
), along with the median ± IQR for each group. No significant difference between the adjusted values was found with a Kruskal–Wallis test (p > 0.05)References
-
- Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, Fleischmann-Struzek C, Machado FR, Reinhart KK, Rowan K, Seymour CW, Watson RS, West TE, Marinho F, Hay SI, Lozano R, Lopez AD, Angus DC, Murray CJL, Naghavi M (2020) Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet 395:200–211 - PMC - PubMed
-
- Venkatesh B, Finfer S, Cohen J, Rajbhandari D, Arabi Y, Bellomo R, Billot L, Correa M, Glass P, Harward M, Joyce C, Li Q, McArthur C, Perner A, Rhodes A, Thompson K, Webb S, Myburgh J, Investigators AT, the Australian-New Zealand Intensive Care Society Clinical Trials G (2018) Adjunctive Glucocorticoid Therapy in Patients with Septic Shock. N Engl J Med 378:797–808 - PubMed
-
- Annane D, Renault A, Brun-Buisson C, Megarbane B, Quenot JP, Siami S, Cariou A, Forceville X, Schwebel C, Martin C, Timsit JF, Misset B, Ali Benali M, Colin G, Souweine B, Asehnoune K, Mercier E, Chimot L, Charpentier C, Francois B, Boulain T, Petitpas F, Constantin JM, Dhonneur G, Baudin F, Combes A, Bohe J, Loriferne JF, Amathieu R, Cook F, Slama M, Leroy O, Capellier G, Dargent A, Hissem T, Maxime V, Bellissant E, Network C-T (2018) Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med 378:809–818 - PubMed
-
- Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, McIntyre L, Ostermann M, Prescott HC, Schorr C, Simpson S, Wiersinga WJ, Alshamsi F, Angus DC, Arabi Y, Azevedo L, Beale R, Beilman G, Belley-Cote E, Burry L, Cecconi M, Centofanti J, Coz Yataco A, De Waele J, Dellinger RP, Doi K, Du B, Estenssoro E, Ferrer R, Gomersall C, Hodgson C, Moller MH, Iwashyna T, Jacob S, Kleinpell R, Klompas M, Koh Y, Kumar A, Kwizera A, Lobo S, Masur H, McGloughlin S, Mehta S, Mehta Y, Mer M, Nunnally M, Oczkowski S, Osborn T, Papathanassoglou E, Perner A, Puskarich M, Roberts J, Schweickert W, Seckel M, Sevransky J, Sprung CL, Welte T, Zimmerman J, Levy M (2021) Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med 47:1181–1247 - PMC - PubMed
-
- Hammond NE, Kumar A, Vijayaraghavan BKT, Arabi YM, Cohen J, Di Tanna GL, Grattan S, van Heerden V, Joynt G, Machado FR, Perner A, Rhodes A, Yeh T, Venkatesh B (2021) Clinician preferences for prescription of corticosteroids in patients with septic shock: an international survey. Crit Care Resusc 23:234–238 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

