Balloon Angioplasty vs Medical Management for Intracranial Artery Stenosis: The BASIS Randomized Clinical Trial
- PMID: 39235816
- PMCID: PMC11378071
- DOI: 10.1001/jama.2024.12829
Balloon Angioplasty vs Medical Management for Intracranial Artery Stenosis: The BASIS Randomized Clinical Trial
Erratum in
-
Errors in Reported Laboratory Values.JAMA. 2024 Dec 24;332(24):2119. doi: 10.1001/jama.2024.24919. JAMA. 2024. PMID: 39602302 Free PMC article. No abstract available.
-
Error in Table.JAMA. 2025 Jun 10;333(22):2025. doi: 10.1001/jama.2025.7552. JAMA. 2025. PMID: 40354164 Free PMC article. No abstract available.
Abstract
Importance: Previous randomized clinical trials did not demonstrate the superiority of endovascular stenting over aggressive medical management for patients with symptomatic intracranial atherosclerotic stenosis (sICAS). However, balloon angioplasty has not been investigated in a randomized clinical trial.
Objective: To determine whether balloon angioplasty plus aggressive medical management is superior to aggressive medical management alone for patients with sICAS.
Design, setting, and participants: A randomized, open-label, blinded end point clinical trial at 31 centers across China. Eligible patients aged 35 to 80 years with sICAS defined as recent transient ischemic attack (<90 days) or ischemic stroke (14-90 days) before enrollment attributed to a 70% to 99% atherosclerotic stenosis of a major intracranial artery receiving treatment with at least 1 antithrombotic drug and/or standard risk factor management were recruited between November 8, 2018, and April 2, 2022 (final follow-up: April 3, 2023).
Interventions: Submaximal balloon angioplasty plus aggressive medical management (n = 249) or aggressive medical management alone (n = 252). Aggressive medical management included dual antiplatelet therapy for the first 90 days and risk factor control.
Main outcomes and measures: The primary outcome was a composite of any stroke or death within 30 days after enrollment or after balloon angioplasty of the qualifying lesion or any ischemic stroke in the qualifying artery territory or revascularization of the qualifying artery after 30 days through 12 months after enrollment.
Results: Among 512 randomized patients, 501 were confirmed eligible (mean age, 58.0 years; 158 [31.5%] women) and completed the trial. The incidence of the primary outcome was lower in the balloon angioplasty group than the medical management group (4.4% vs 13.5%; hazard ratio, 0.32 [95% CI, 0.16-0.63]; P < .001). The respective rates of any stroke or all-cause death within 30 days were 3.2% and 1.6%. Beyond 30 days through 1 year after enrollment, the rates of any ischemic stroke in the qualifying artery territory were 0.4% and 7.5%, respectively, and revascularization of the qualifying artery occurred in 1.2% and 8.3%, respectively. The rate of symptomatic intracranial hemorrhage in the balloon angioplasty and medical management groups was 1.2% and 0.4%, respectively. In the balloon angioplasty group, procedural complications occurred in 17.4% of patients and arterial dissection occurred in 14.5% of patients.
Conclusions and relevance: In patients with sICAS, balloon angioplasty plus aggressive medical management, compared with aggressive medical management alone, statistically significantly lowered the risk of a composite outcome of any stroke or death within 30 days or an ischemic stroke or revascularization of the qualifying artery after 30 days through 12 months. The findings suggest that balloon angioplasty plus aggressive medical management may be an effective treatment for sICAS, although the risk of stroke or death within 30 days of balloon angioplasty should be considered in clinical practice.
Trial registration: ClinicalTrials.gov Identifier: NCT03703635.
Conflict of interest statement
Figures

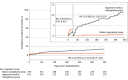
Comment in
-
Is Balloon Angioplasty the Future for Intracranial Stenosis?JAMA. 2024 Oct 1;332(13):1055-1056. doi: 10.1001/jama.2024.13547. JAMA. 2024. PMID: 39235792 No abstract available.
References
-
- Zaidat OO, Fitzsimmons BF, Woodward BK, et al. ; VISSIT Trial Investigators . Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA. 2015;313(12):1240-1248. doi: 10.1001/jama.2015.1693 - DOI - PubMed
-
- Gao P, Wang T, Wang D, et al. ; CASSISS Trial Investigators . Effect of stenting plus medical therapy vs medical therapy alone on risk of stroke and death in patients with symptomatic intracranial stenosis: the CASSISS randomized clinical trial. JAMA. 2022;328(6):534-542. doi: 10.1001/jama.2022.12000 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

