Aryl Hydrocarbon Receptor Pathway Augments Peritoneal Fibrosis in a Murine CKD Model Exposed to Peritoneal Dialysate
- PMID: 39235862
- PMCID: PMC11441816
- DOI: 10.34067/KID.0000000000000516
Aryl Hydrocarbon Receptor Pathway Augments Peritoneal Fibrosis in a Murine CKD Model Exposed to Peritoneal Dialysate
Abstract
Key Points:
CKD and high glucose–containing peritoneal dialysate alter peritoneal membrane contributing to peritoneal dialysis failure, with a poorly understood mechanism.
CKD milieu activates the aryl hydrocarbon receptor pathway in the subperitoneal vasculature, increasing the peritoneal fibrosis and collagen deposition in humans and mice.
An aryl hydrocarbon receptor inhibitor mitigates CKD and peritoneal dialysis–mediated peritoneal fibrosis, collagen deposition, and vasculogenesis in a mouse model.
Background: CKD is a proinflammatory and profibrotic condition and can independently alter the peritoneal membrane structure. Peritoneal dialysis (PD) results in profound alterations in the peritoneal membrane. The mechanisms contributing to the alterations of the peritoneal membrane structure in CKD milieu, along with PD, are poorly understood.
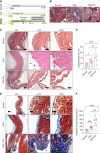
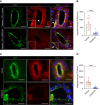
Methods: Here, we show that human CKD induces peritoneal membrane thickening, fibrosis, and collagen deposition and activates the aryl hydrocarbon receptor (AHR) pathway in the subperitoneal vasculature. Leveraging a novel model of PD in CKD mice, we confirm these CKD-induced changes in the peritoneal membrane, which are exacerbated on exposure to the peritoneal dialysate. Peritoneal dialysate further augmented the AHR activity in endothelial cells of peritoneal microvasculature in CKD mice.
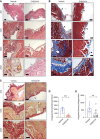
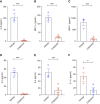
Results: Treatment of CKD mice with an AHR inhibitor in peritoneal dialysate for 2 weeks resulted in a seven-fold reduction in AHR expression in the endothelial cells of subperitoneal capillaries, a five-fold decrease in subperitoneal space, and a nine-fold decrease in fibrosis and collagen deposition compared with vehicle-treated CKD mice. AHR inhibition reduced inflammation, subperitoneal neovascular areas, and its downstream target, tissue factor. The AHR inhibitor treatment normalized the peritoneal dialysate-induced proinflammatory and profibrotic cytokines, such as IL-6, monocyte chemoattractant protein-1, and macrophage inflammatory protein 1 levels, in CKD mice.
Conclusions: This study uncovers the activation of the AHR-cytokine axis in the endothelial cells of subperitoneal vessels in humans and mice with CKD, which is likely to prime the peritoneal membrane to peritoneal dialysate–mediated alterations. This study supports further exploration of AHR as a potential therapeutic target to preserve the structural and functional integrity of the peritoneal membrane in PD.
Conflict of interest statement
Disclosure forms, as provided by each author, are available with the online version of the article at
Figures



References
-
- Centers for Disease Control and Prevention. Chronic Kidney Disease in the United States, 2019. US Department of Health and Human Services, Centers for Disease Control and Prevention; 2019.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

