Acquired immunostimulatory phenotype of migratory CD103+ DCs promotes alloimmunity following corneal transplantation
- PMID: 39235864
- PMCID: PMC11530131
- DOI: 10.1172/jci.insight.182469
Acquired immunostimulatory phenotype of migratory CD103+ DCs promotes alloimmunity following corneal transplantation
Abstract
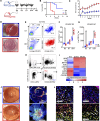
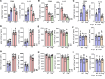
After transplantation, Th1-mediated immune rejection is the predominant cause of graft failure. Th1 cell sensitization occurs through complex and context-dependent interaction among antigen-presenting cell subsets, particularly CD11b+ DCs (DC2) and CD103+ DCs (DC1). This interaction necessitates further investigation in the context of transplant immunity. We used well-established preclinical models of corneal transplantation and identified distinct roles of migratory CD103+ DC1 in influencing the outcomes of the grafted tissue. In recipients with uninflamed corneal beds, migratory CD103+ DC1 demonstrate a tolerogenic phenotype that modulates the immunogenic capacity of CD11b+ DC2 primarily mediated by IL-10, suppressing alloreactive CD4+ Th1 cells via the PD-L1/PD-1 pathway and enhancing Treg-mediated tolerance via αvβ8 integrin-activated TGF-β1, thus facilitating graft survival. Conversely, in recipients with inflamed and vascularized corneal beds, IFN-γ produced by CD4+ Th1 cells induced migratory CD103+ DC1 to adopt an immunostimulatory phenotype, characterized by the downregulation of regulatory markers, including αvβ8 integrin and IL-10, and the upregulation of IL-12 and costimulatory molecules CD80/86, resulting in graft failure. The adoptive transfer of ex vivo induced tolerogenic CD103+ DC1 (iDC1) effectively inhibited Th1 polarization and preserved the tolerogenic phenotype of their physiological counterparts. Collectively, our findings underscore the essential role played by CD103+ DC1 in modulating host alloimmune responses.
Keywords: Dendritic cells; Immunology; Ophthalmology; Organ transplantation; Tolerance.
Figures





References
-
- Mathias CB, McAleer JP. Transplantation: Immunologic principles and pharmacologic agents. In: Mathias CB, et al, eds. Pharmacology of Immunotherapeutic Drugs. Springer; 2019:251–274.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

