Hepatocellular adenoma update: diagnosis, molecular classification, and clinical course
- PMID: 39235933
- PMCID: PMC11491668
- DOI: 10.1093/bjr/tqae180
Hepatocellular adenoma update: diagnosis, molecular classification, and clinical course
Abstract
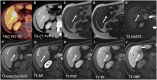
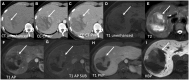
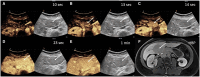
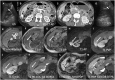
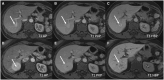
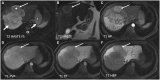
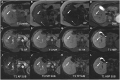
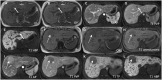
Hepatocellular adenomas (HCA) are acquired focal liver lesions, that occur mainly in young-to-middle-aged women who are on long-term estrogen-containing contraceptives or young men after prolonged use of anabolic steroids. Furthermore, distinct underlying diseases, such as obesity, metabolic dysfunction-associated steatotic liver disease, glycogen storage disease, etc. are considered risk factors. The 2017 Bordeaux classification, in particular Nault et al, divided HCAs into eight subtypes according to their pheno- and genotypic characteristics. This includes HCAs with hepatocyte-nuclear-factor (HNF1-alpha mutation), HCAs with β-catenin mutation, and HCAs without either of these genetic mutations, which are further subdivided into HCAs with and without inflammatory cells. HCAs should no longer be classified as purely benign without histologic workup since three of the eight subtypes are considered high-risk lesions, requiring adequate management: malignant transformation of the pure (ßex3-HCA) and mixed inflammatory/β-catenin exon 3 (ßex3-IHCA) adenomas, as well as potential bleeding of the sonic hedgehog HCA and pure (ßex7/8-HCA) and mixed inflammatory/β-catenin exon 7/8 (ßex7/8-IHCA). Elective surgery is recommended for any HCA in a male, or for any HCA exceeding 5 cm. Although MRI can classify up to 80% of adenomas, if findings are equivocal, biopsy remains the reference standard for adenoma subtype.
Keywords: MRI; contrast agents; hepatocellular adenoma; liver; molecular imaging.
© The Author(s) 2024. Published by Oxford University Press on behalf of the British Institute of Radiology.
Conflict of interest statement
P.-L.: nothing to disclose. A. B.-S.: consulting fees from Bayer; honoraria for lectures for Novartis, Siemens, and Bayer. N. B.: nothing to disclose. S. B.-S.: nothing to disclose. J. C. H.: nothing to disclose.G. B.: honoraria for lectures for GE Healthcare, Bayer, Bracco, and Guerbet. V. P.: no COI related to this topic. V. V.: no COI related to this topic.
Figures




References
-
- European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity. EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388-1402. - PubMed
-
- Nault J-C, Couchy G, Balabaud C, et al. ; GENTHEP Investigators. Molecular classification of hepatocellular adenoma associates with risk factors, bleeding, and malignant transformation. Gastroenterology. 2017;152(4):880-894 e6. - PubMed
-
- Shreenath AP, Kahloon A. Hepatic adenoma. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Arslan Kahloon declares no relevant financial relationships with ineligible companies. 2023.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

