Modelling neurocardiac physiology and diseases using human pluripotent stem cells: current progress and future prospects
- PMID: 39235952
- PMCID: PMC11955871
- DOI: 10.1113/JP286416
Modelling neurocardiac physiology and diseases using human pluripotent stem cells: current progress and future prospects
Abstract
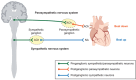
Throughout our lifetime the heart executes cycles of contraction and relaxation to meet the body's ever-changing metabolic needs. This vital function is continuously regulated by the autonomic nervous system. Cardiovascular dysfunction and autonomic dysregulation are also closely associated; however, the degrees of cause and effect are not always readily discernible. Thus, to better understand cardiovascular disorders, it is crucial to develop model systems that can be used to study the neurocardiac interaction in healthy and diseased states. Human pluripotent stem cell (hiPSC) technology offers a unique human-based modelling system that allows for studies of disease effects on the cells of the heart and autonomic neurons as well as of their interaction. In this review, we summarize current understanding of the embryonic development of the autonomic, cardiac and neurocardiac systems, their regulation, as well as recent progress of in vitro modelling systems based on hiPSCs. We further discuss the advantages and limitations of hiPSC-based models in neurocardiac research.
Keywords: autonomic nervous system; cardiology; disease modelling; human pluripotent stem cell; neurocardiology.
© 2024 The Author(s). The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
All authors declare that there are no competing interests.
Figures
References
-
- Aalders, J. , Léger, L. , van der Meeren, L. , Sinha, S. , Skirtach, A G. , De Backer, J. , & van Hengel, J. (2024). Three‐dimensional co‐culturing of stem cell‐derived cardiomyocytes and cardiac fibroblasts reveals a role for both cell types in Marfan‐related cardiomyopathy. Matrix Biology, 126, 14–24. - PubMed
-
- Ahmad, F. S. , Jin, Y. , Grassam‐Rowe, A. , Zhou, Y. , Yuan, M. , Fan, X. , Zhou, R. , Mu‐U‐Min, R. , O'shea, C. , Ibrahim, A M. , Hyder, W. , Aguib, Y. , Yacoub, M. , Pavlovic, D. , Zhang, Y. , Tan, X. , Lei, M. , & Terrar, D. A. (2023). Generation of cardiomyocytes from human‐induced pluripotent stem cells resembling atrial cells with ability to respond to adrenoceptor agonists. Philosophical Transactions of the Royal Society of London‐Series B: Biological Sciences, 378(1879), 20220312. - PMC - PubMed
-
- Ajijola, O. A. , Chatterjee, N. A. , Gonzales, M. J. , Gornbein, J. , Liu, K. , Li, D. , Paterson, D. J. , Shivkumar, K. , Singh, J. P. , & Herring, N. (2020). Coronary sinus neuropeptide Y levels and adverse outcomes in patients with stable chronic heart failure. Journal of the American Medical Association Cardiology, 5(3), 318–325. - PMC - PubMed
-
- Arslanova, A. , Shafaattalab, S. , Ye, K. , Asghari, P. , Lin, L. , Kim, B. , Roston, T. M. , Hove‐Madsen, L. , Van Petegem, F. , Sanatani, S. , Moore, E. , Lynn, F. , Søndergaard, M. , Luo, Y. , Chen, S. R. W. , & Tibbits, G. F. (2021). Using hiPSC‐CMs to examine mechanisms of catecholaminergic polymorphic ventricular tachycardia. Current Protocol, 1(12), e320. - PubMed
-
- Avior, Y. , Sagi, I. , & Benvenisty, N. (2016). Pluripotent stem cells in disease modelling and drug discovery. Nature Reviews Molecular Cell Biology, 17(3), 170–182. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources