RETRACTED: Curcumin alleviates osteoarthritis in mice by suppressing osteoclastogenesis in subchondral bone via inhibiting NF-κB/JNK signaling pathway
- PMID: 39236007
- PMCID: PMC11376521
- DOI: 10.1371/journal.pone.0309807
RETRACTED: Curcumin alleviates osteoarthritis in mice by suppressing osteoclastogenesis in subchondral bone via inhibiting NF-κB/JNK signaling pathway
Retraction in
-
Retraction: Curcumin alleviates osteoarthritis in mice by suppressing osteoclastogenesis in subchondral bone via inhibiting NF-κB/JNK signaling pathway.PLoS One. 2026 Jan 8;21(1):e0340040. doi: 10.1371/journal.pone.0340040. eCollection 2026. PLoS One. 2026. PMID: 41505380 Free PMC article. No abstract available.
Abstract
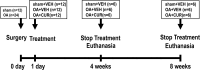
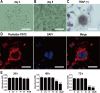
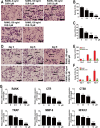
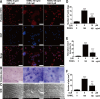
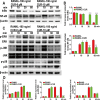
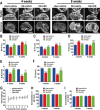
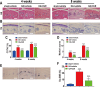
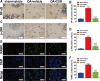
This study explored the mechanism of curcumin (CUR) suppressing osteoclastogenesis and evaluated its effects on osteoarthritis (OA) mouse. Bone marrow-derived macrophages were isolated as osteoclast precursors. In the presence or absence of CUR, cell proliferation was detected by CCK-8, osteoclastogenesis was detected by tartrate-resistant acid phosphatase (TRAP) staining, F-actin rings formation was detected by immunofluorescence, bone resorption was detected by bone slices, IκBα, nuclear factor kappa-B (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways were detected using western blot, osteoclastogenesis-related gens were measured using quantitative polymerase chain reaction. A knee OA mouse model was designed by destabilizing the medial meniscus (DMM). Thirty-six male mice were divided into sham+vehicle, OA+vehicle, and OA+CUR groups. Mice were administered with or without CUR at 25 mg/kg/d from the first post-operative day until sacrifice. After 4 and 8 weeks of OA induction, micro-computed tomography was performed to analyze microstructure changes in subchondral bone, hematoxylin and eosin staining was performed to calculate the thickness of the calcified and hyaline cartilage layers, toluidine blue O staining was performed to assess the degenerated cartilage, TRAP-stained osteoclasts were counted, and NF-κB, phosphorylated Jun N-terminal Kinases (p-JNK), and receptor activator of nuclear factor κB ligand (RANKL) were detected using immunohistochemistry. CUR suppressed osteoclastogenesis and bone resorption without cytotoxicity. CUR restrained RANKL-induced activation of NF-κB, p-JNK and up-regulation of osteoclastogenesis-related genes. CUR delayed cartilage degeneration by suppressing osteoclastogenesis and bone resorption in early OA. The mechanism of CUR inhibiting osteoclastogenesis might be associated with NF-κB/JNK signaling pathway, indicating a novel strategy for OA treatment.
Copyright: © 2024 Ding et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- G P, MJ T. Osteoarthritis year in review 2020: epidemiology & therapy. OSTEOARTHR CARTILAGE. 2021;29(2):180–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

