Inositol trisphosphate and ryanodine receptor signaling distinctly regulate neurite pathfinding in response to engineered micropatterned surfaces
- PMID: 39236043
- PMCID: PMC11376539
- DOI: 10.1371/journal.pone.0308389
Inositol trisphosphate and ryanodine receptor signaling distinctly regulate neurite pathfinding in response to engineered micropatterned surfaces
Abstract
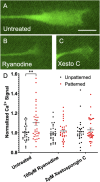
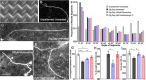
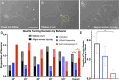
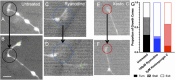
Micro and nanoscale patterning of surface features and biochemical cues have emerged as tools to precisely direct neurite growth into close proximity with next generation neural prosthesis electrodes. Biophysical cues can exert greater influence on neurite pathfinding compared to the more well studied biochemical cues; yet the signaling events underlying the ability of growth cones to respond to these microfeatures remain obscure. Intracellular Ca2+ signaling plays a critical role in how a growth cone senses and grows in response to various cues (biophysical features, repulsive peptides, chemo-attractive gradients). Here, we investigate the role of inositol triphosphate (IP3) and ryanodine-sensitive receptor (RyR) signaling as sensory neurons (spiral ganglion neurons, SGNs, and dorsal root ganglion neurons, DRGNs) pathfind in response to micropatterned substrates of varied geometries. We find that IP3 and RyR signaling act in the growth cone as they navigate biophysical cues and enable proper guidance to biophysical, chemo-permissive, and chemo-repulsive micropatterns. In response to complex micropatterned geometries, RyR signaling appears to halt growth in response to both topographical features and chemo-repulsive cues. IP3 signaling appears to play a more complex role, as growth cones appear to sense the microfeatures in the presence of xestospongin C but are unable to coordinate turning in response to them. Overall, key Ca2+ signaling elements, IP3 and RyR, are found to be essential for SGNs to pathfind in response to engineered biophysical and biochemical cues. These findings inform efforts to precisely guide neurite regeneration for improved neural prosthesis function, including cochlear implants.
Copyright: © 2024 Vecchi et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Intracellular calcium and cyclic nucleotide levels modulate neurite guidance by microtopographical substrate features.J Biomed Mater Res A. 2016 Aug;104(8):2037-48. doi: 10.1002/jbm.a.35738. Epub 2016 Apr 19. J Biomed Mater Res A. 2016. PMID: 27062708 Free PMC article.
-
The geometry of photopolymerized topography influences neurite pathfinding by directing growth cone morphology and migration.bioRxiv [Preprint]. 2023 Aug 29:2023.08.28.555111. doi: 10.1101/2023.08.28.555111. bioRxiv. 2023. Update in: J Neural Eng. 2024 Apr 04;21(2). doi: 10.1088/1741-2552/ad38dc. PMID: 37693432 Free PMC article. Updated. Preprint.
-
The geometry of photopolymerized topography influences neurite pathfinding by directing growth cone morphology and migration.J Neural Eng. 2024 Apr 4;21(2):026027. doi: 10.1088/1741-2552/ad38dc. J Neural Eng. 2024. PMID: 38547528 Free PMC article.
-
Photopolymerized Microfeatures Guide Adult Spiral Ganglion and Dorsal Root Ganglion Neurite Growth.Otol Neurotol. 2018 Jan;39(1):119-126. doi: 10.1097/MAO.0000000000001622. Otol Neurotol. 2018. PMID: 29227456 Free PMC article.
-
Interaction of micropatterned topographical and biochemical cues to direct neurite growth from spiral ganglion neurons.Hear Res. 2021 Sep 15;409:108315. doi: 10.1016/j.heares.2021.108315. Epub 2021 Jul 21. Hear Res. 2021. PMID: 34343850 Free PMC article.
References
-
- Schmidbauer D, Fink S, Rousset F, Löwenheim H, Senn P, Glueckert R. Closing the Gap between the Auditory Nerve and Cochlear Implant Electrodes: Which Neurotrophin Cocktail Performs Best for Axonal Outgrowth and Is Electrical Stimulation Beneficial? International Journal of Molecular Sciences. 2023;24(3):2013. doi: 10.3390/ijms24032013 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

