Transition to dolutegravir-based ART in 35 low- and middle-income countries: a global survey of HIV care clinics
- PMID: 39236112
- PMCID: PMC11562491
- DOI: 10.1097/QAD.0000000000004007
Transition to dolutegravir-based ART in 35 low- and middle-income countries: a global survey of HIV care clinics
Abstract
Objective: We studied the transition to dolutegravir-containing antiretroviral therapy (ART) at HIV treatment clinics within the International epidemiology Databases to Evaluate AIDS (IeDEA).
Design: Site-level survey conducted in 2020-2021 among HIV clinics in low- and middle-income countries (LMICs).
Methods: We assessed the status of dolutegravir rollout and viral load and drug resistance testing practices for persons on ART switching to dolutegravir-based regimens. We used generalized estimating equations to assess associations between clinic rollout of both first- and second-line dolutegravir-based ART regimens (dual rollout) and site-level factors.
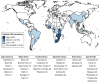
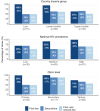
Results: Of 179 surveyed clinics, 175 (98%) participated; 137 (78%) from Africa, 30 (17%) from the Asia-Pacific, and 8 (5%) from Latin America. Most clinics (80%) were in low- or lower-middle-income countries, and there were a mix of primary-, secondary- and tertiary-level clinics. Ninety percent reported rollout of first-line dolutegravir, 59% of second-line, 94% of first- or second-line and 55% of dual rollout. The adjusted odds of dual rollout were higher among tertiary-level [adjusted odds ratio (aOR) 4.00; 95% confidence interval (CI) 1.39-11.47] and secondary-level clinics (aOR 3.66; 95% CI 2.19-6.11) than in primary-level clinics. Over half (59%) of clinics that introduced first- or second-line dolutegravir-based ART required recent viral load testing before switching to dolutegravir, and 15% performed genotypic resistance testing at switch.
Conclusions: Dolutegravir-based ART was rolled out at nearly all IeDEA clinics in LMICs, yet many switched persons to dolutegravir without recent viral load testing and drug resistance testing was rarely performed. Without such testing, drug resistance among persons switching to dolutegravir may go undetected.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
There are no conflicts of interest.
Figures

References
-
- Venter WDF, Sokhela S, Simmons B, Moorhouse M, Fairlie L, Mashabane N, et al. Dolutegravir with emtricitabine and tenofovir alafenamide or tenofovir disoproxil fumarate versus efavirenz, emtricitabine, and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection (ADVANCE): week 96 results from a randomised, phase 3, n. Lancet HIV 2020; 7:e666–e676. - PubMed
-
- Calmy A, Tovar Sanchez T, Kouanfack C, Mpoudi-Etame M, Leroy S, Perrineau S, et al. Dolutegravir-based and low-dose efavirenz-based regimen for the initial treatment of HIV-1 infection (NAMSAL): week 96 results from a two-group, multicentre, randomised, open label, phase 3 noninferiority trial in Cameroon. Lancet HIV 2020; 7:e677–e687. - PubMed
-
- Kanters S, Vitoria M, Doherty M, Socias ME, Ford N, Forrest JI, et al. Comparative efficacy and safety of first-line antiretroviral therapy for the treatment of HIV infection: a systematic review and network meta-analysis. Lancet HIV 2016; 3:e510–e520. - PubMed
-
- Kanters S, Vitoria M, Zoratti M, Doherty M, Penazzato M, Rangaraj A, et al. Comparative efficacy, tolerability and safety of dolutegravir and efavirenz 400 mg among antiretroviral therapies for first-line HIV treatment: a systematic literature review and network meta-analysis. EClinicalMedicine 2020; 28:100573. - PMC - PubMed
-
- Walmsley S, Baumgarten A, Berenguer J, Felizarta F, Florence E, Khuong-Josses MA, et al. Dolutegravir plus abacavir/lamivudine for the treatment of HIV-1 infection in antiretroviral therapy-naive patients: week 96 and week 144 results from the SINGLE randomized clinical trial. J Acquir Immune Defic Syndr 2015; 70:515–519. - PMC - PubMed

