The use of ectopic volar fibroblasts to modify skin identity
- PMID: 39236183
- PMCID: PMC11457755
- DOI: 10.1126/science.adi1650
The use of ectopic volar fibroblasts to modify skin identity
Abstract
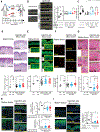
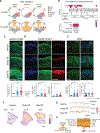
Skin identity is controlled by intrinsic features of the epidermis and dermis and their interactions. Modifying skin identity has clinical potential, such as the conversion of residual limb and stump (nonvolar) skin of amputees to pressure-responsive palmoplantar (volar) skin to enhance prosthesis use and minimize skin breakdown. Greater keratin 9 (KRT9) expression, higher epidermal thickness, keratinocyte cytoplasmic size, collagen length, and elastin are markers of volar skin and likely contribute to volar skin resiliency. Given fibroblasts' capacity to modify keratinocyte differentiation, we hypothesized that volar fibroblasts influence these features. Bioprinted skin constructs confirmed the capacity of volar fibroblasts to induce volar keratinocyte features. A clinical trial of healthy volunteers demonstrated that injecting volar fibroblasts into nonvolar skin increased volar features that lasted up to 5 months, highlighting a potential cellular therapy.
Conflict of interest statement
Figures


Comment in
-
Transplanted fibroblasts take the pressure.Science. 2024 Sep 6;385(6713):1047-1048. doi: 10.1126/science.adr9294. Epub 2024 Sep 5. Science. 2024. PMID: 39236196
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

