Mechanical confinement prevents ectopic platelet release
- PMID: 39236232
- PMCID: PMC11420179
- DOI: 10.1073/pnas.2407829121
Mechanical confinement prevents ectopic platelet release
Abstract
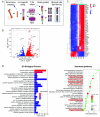
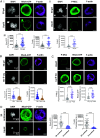
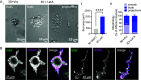
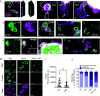
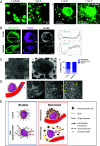
Blood platelets are produced by megakaryocytes (MKs), their parent cells, which are in the bone marrow. Once mature, MK pierces through the sinusoid vessel, and the initial protrusion further elongates as proplatelet or buds to release platelets. The mechanisms controlling the decision to initiate proplatelet and platelet formation are unknown. Here, we show that the mechanical properties of the microenvironment prevent proplatelet and platelet release in the marrow stroma while allowing this process in the bloodstream. Loss of marrow confinement following myelosuppression led to inappropriate proplatelet and platelet release into the extravascular space. We further used an inert viscoelastic hydrogel to evaluate the impact of compressive stress. Transcriptional analysis showed that culture in three-dimensional gel induced upregulation of genes related to the Rho-GTPase pathway. We found higher Rho-GTPase activation, myosin light chain phosphorylation and F-actin under mechanical constraints while proplatelet formation was inhibited. The use of latrunculin-A to decrease F-actin promoted microtubule-dependent budding and proplatelet extension inside the gel. Additionally, ex vivo exposure of intact bone marrow to latrunculin-A triggered proplatelet extensions in the interstitial space. In vivo, this confinement-mediated high intracellular tension is responsible for the formation of the peripheral zone, a unique actin-rich structure. Cytoskeleton reorganization induces the disappearance of the peripheral zone upon reaching a liquid milieu to facilitate proplatelet and platelet formation. Hence, our data provide insight into the mechanisms preventing ectopic platelet release in the marrow stroma. Identifying such pathways is especially important for understanding pathologies altering marrow mechanics such as chemotherapy or myelofibrosis.
Keywords: 3D culture; cytoskeleton; mechanical confinement; megakaryocyte; myelosuppression.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

