Single-molecule force spectroscopy reveals intra- and intermolecular interactions of Caenorhabditis elegans HMP-1 during mechanotransduction
- PMID: 39236238
- PMCID: PMC11406289
- DOI: 10.1073/pnas.2400654121
Single-molecule force spectroscopy reveals intra- and intermolecular interactions of Caenorhabditis elegans HMP-1 during mechanotransduction
Abstract
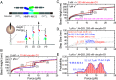
The Caenorhabditis elegans HMP-2/HMP-1 complex, akin to the mammalian [Formula: see text]-catenin-[Formula: see text]-catenin complex, serves as a critical mechanosensor at cell-cell adherens junctions, transducing tension between HMR-1 (also known as cadherin in mammals) and the actin cytoskeleton. Essential for embryonic development and tissue integrity in C. elegans, this complex experiences tension from both internal actomyosin contractility and external mechanical microenvironmental perturbations. While offering a valuable evolutionary comparison to its mammalian counterpart, the impact of tension on the mechanical stability of HMP-1 and HMP-2/HMP-1 interactions remains unexplored. In this study, we directly quantified the mechanical stability of full-length HMP-1 and its force-bearing modulation domains (M1-M3), as well as the HMP-2/HMP-1 interface. Notably, the M1 domain in HMP-1 exhibits significantly higher mechanical stability than its mammalian analog, attributable to interdomain interactions with M2-M3. Introducing salt bridge mutations in the M3 domain weakens the mechanical stability of the M1 domain. Moreover, the intermolecular HMP-2/HMP-1 interface surpasses its mammalian counterpart in mechanical stability, enabling it to support the mechanical activation of the autoinhibited M1 domain for mechanotransduction. Additionally, the phosphomimetic mutation Y69E in HMP-2 weakens the mechanical stability of the HMP-2/HMP-1 interface, compromising the force-transmission molecular linkage and its associated mechanosensing functions. Collectively, these findings provide mechanobiological insights into the C. elegans HMP-2/HMP-1 complex, highlighting the impact of salt bridges on mechanical stability in [Formula: see text]-catenin and demonstrating the evolutionary conservation of the mechanical switch mechanism activating the HMP-1 modulation domain for protein binding at the single-molecule level.
Keywords: HMP-1; HMP-2; magnetic tweezers; salt bridges.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures






References
-
- Le S., Yu M., Yan J., Mechanical regulation of tension-transmission supramolecular linkages. Curr. Opin. Solid State Mater. Sci. 25, 100895 (2021).
-
- Charras G., Yap A. S., Tensile forces and mechanotransduction at cell–cell junctions. Curr. Biol. 28, R445–R457 (2018). - PubMed
-
- Hirano Y., Amano Y., Yonemura S., Hakoshima T., The force-sensing device region of -catenin is an intrinsically disordered segment in the absence of intramolecular stabilization of the autoinhibitory form. Genes Cells 23, 370–385 (2018). - PubMed
-
- Yao M., et al. , Force-dependent conformational switch of -catenin controls vinculin binding. Nat. Commun. 5, 4525 (2014). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

