Lentiviral Gene Therapy for Cystic Fibrosis: A Promising Approach and First-in-Human Trial
- PMID: 39236265
- PMCID: PMC11716034
- DOI: 10.1164/rccm.202402-0389CI
Lentiviral Gene Therapy for Cystic Fibrosis: A Promising Approach and First-in-Human Trial
Abstract
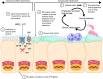
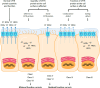
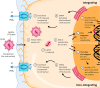
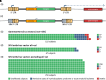
Cystic fibrosis (CF) is a genetic disease caused by mutations in the CFTR (cystic fibrosis transmembrane conductance regulator) gene. Although CF is a multiorgan disease, the leading causes of morbidity and mortality are related to progressive lung disease. Current understanding of the effects of the broad spectrum of CFTR mutations on CFTR function has allowed for the development of CFTR modulator therapies. Despite the remarkable impact that these therapies have had, there remains a significant proportion of people with CF (estimated at 10-15% of the global CF population) who are genetically ineligible for, or intolerant of, current CFTR-targeting therapies and whose therapeutic needs remain unmet. Inhaled genetic therapies offer the prospect of addressing the unmet pulmonary treatment need in people with CF, with several approaches, including gene addition therapy (the focus of this review), RNA-based therapies, antisense oligonucleotides, and gene editing, being explored. Various nonviral and viral vectors have been investigated for CF gene addition therapy for mutation-agnostic restoration of CFTR function in the lungs. Lentiviral vectors offer the prospect of highly efficient and long-lasting gene expression, and the potential to be safely and, in contrast to other commonly used viral vectors, effectively redosed. A third-generation lentiviral vector pseudotyped with Sendai virus F and HN envelope proteins (rSIV.F/HN) has been developed for the treatment of CF. Promising preclinical results support the progression of this vector carrying a full-length CFTR transgene (BI 3720931) into a first-in-human clinical trial expected to begin in 2024.
Keywords: CFTR; genetic therapy; integrating vectors; lentivirus; mutation-agnostic treatment.
Figures
Comment in
-
The Road Less Traveled: Slow but Steady Progress Toward Cystic Fibrosis Gene Therapy by the UK Respiratory Gene Therapy Consortium.Am J Respir Crit Care Med. 2024 Dec 15;210(12):1387-1389. doi: 10.1164/rccm.202408-1516ED. Am J Respir Crit Care Med. 2024. PMID: 39265185 Free PMC article. No abstract available.
References
-
- Shteinberg M, Haq IJ, Polineni D, Davies JC. Cystic fibrosis. Lancet . 2021;397:2195–2211. - PubMed
-
- Ong T, Ramsey BW. Cystic fibrosis: a review. JAMA. 2023;329:1859–1871. - PubMed
-
- Guo J, Garratt A, Hill A. Worldwide rates of diagnosis and effective treatment for cystic fibrosis. J Cyst Fibros . 2022;21:456–462. - PubMed
-
- Stutts MJ, Canessa CM, Olsen JC, Hamrick M, Cohn JA, Rossier BC, et al. CFTR as a cAMP-dependent regulator of sodium channels. Science . 1995;269:847–850. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

