From guidelines to clinical practice in care for ischaemic stroke patients: A systematic review and expert opinion
- PMID: 39236303
- PMCID: PMC11554874
- DOI: 10.1111/ene.16417
From guidelines to clinical practice in care for ischaemic stroke patients: A systematic review and expert opinion
Abstract
Background and purpose: Guidelines help physicians to provide optimal care for stroke patients, but implementation is challenging due to the quantity of recommendations. Therefore a practical overview related to applicability of recommendations can be of assistance.
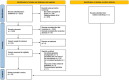
Methods: A systematic review was performed on ischaemic stroke guidelines published in scientific journals, covering the whole acute care process for patients with ischaemic stroke. After data extraction, experts rated the recommendations on dimensions of applicability, that is, actionability, feasibility and validity, on a 9-point Likert scale. Agreement was defined as a score of ≥8 by ≥80% of the experts.
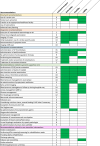
Results: Eighteen articles were identified and 48 recommendations were ultimately extracted. Papers were included only if they described the whole acute care process for patients with ischaemic stroke. Data extraction and analysis revealed variation in terms of both content and comprehensiveness of this description. Experts reached agreement on 34 of 48 (70.8%) recommendations in the dimension actionability, for 16 (33.3%) in feasibility and for 15 (31.3%) in validity. Agreement on all three dimensions was reached for seven (14.6%) recommendations: use of a stroke unit, exclusion of intracerebral haemorrhage as differential diagnosis, administration of intravenous thrombolysis, performance of electrocardiography/cardiac evaluation, non-invasive vascular examination, deep venous thrombosis prophylaxis and administration of statins if needed.
Discussion and conclusion: Substantial variation in agreement was revealed on the three dimensions of the applicability of recommendations. This overview can guide stroke physicians in improving the care process and removing barriers where implementation may be hampered by validity and feasibility.
Keywords: expert testimony; guidelines; ischaemic stroke; quality improvement; systematic review.
© 2024 The Author(s). European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
Dr. Sandset reports other from Bayer, other from AstraZeneca, other from BMS, outside the submitted work; Dr. Thomalla reports personal fees from Acandis, personal fees from Alexion, personal fees from Amarin, personal fees from Astra Zeneca, personal fees from Bayer, personal fees from Boeringer Ingelheim, personal fees from Daiichi Sankyo, personal fees from Stryker,outside the submitted work; Dr. Sacco reports personal fees from Abbvie, personal fees from Novartis, personal fees from Lundbeck, personal fees from Pfizer, personal fees from Boheringer, personal fees from Teva, personal fees from Lilly,outside the submitted work; Dr. Fischer participates in an advisory board for AstraZeneca (former Alexion/Portola), Boehringer Ingelheim, Biogen, AbbVie and Acthera (fees paid to institution); member of a clinical event committee (CEC) of the COATING study (Phenox) and member of the data and safety monitoring committee (DSMB) of the TITAN, LATE_MT and IN EXTREMIS trials; president of the Swiss Neurological Society, president‐elect of the European Stroke Organisation. The other authors have nothing to disclose.
Figures
References
-
- Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early Management of Patients with Acute Ischemic Stroke: 2019 update to the 2018 guidelines for the early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344‐e418. doi: 10.1161/str.0000000000000211 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

