Neuromuscular impairment at different stages of human sarcopenia
- PMID: 39236304
- PMCID: PMC11446718
- DOI: 10.1002/jcsm.13531
Neuromuscular impairment at different stages of human sarcopenia
Abstract
Background: Degeneration of the motoneuron and neuromuscular junction (NMJ) and loss of motor units (MUs) contribute to age-related muscle wasting and weakness associated with sarcopenia. However, these features have not been comprehensively investigated in humans. This study aimed to compare neuromuscular system integrity and function at different stages of sarcopenia, with a particular focus on NMJ stability and MU properties.
Methods: We recruited 42 young individuals (Y) (aged 25.98 ± 4.6 years; 57% females) and 88 older individuals (aged 75.9 ± 4.7 years; 55% females). The older group underwent a sarcopenia screening according to the revised guidelines of the European Working Group on Sarcopenia in Older People 2. In all groups, knee extensor muscle force was evaluated by isometric dynamometry, muscle morphology by ultrasound and MU potential properties by intramuscular electromyography (iEMG). MU number estimate (iMUNE) and blood samples were obtained. Muscle biopsies were collected in a subgroup of 16 Y and 52 older participants.
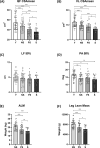
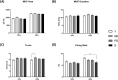
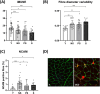
Results: Thirty-nine older individuals were non-sarcopenic (NS), 31 pre-sarcopenic (PS) and 18 sarcopenic (S). A gradual decrease in quadriceps force, cross-sectional area and appendicular lean mass was observed across the different stages of sarcopenia (for all P < 0.0001). Handgrip force and the Short Physical Performance Battery score also showed a diminishing trend. iEMG analyses revealed elevated near fibre segment jitter in NS, PS and S compared with Y (Y vs. NS and S: P < 0.0001; Y vs. PS: P = 0.0169), suggestive of age-related impaired NMJ transmission. Increased C-terminal agrin fragment (P < 0.0001) and altered caveolin 3 protein expression were consistent with age-related NMJ instability in all the older groups. The iMUNE was lower in all older groups (P < 0.0001), confirming age-related loss of MUs. An age-related increase in MU potential complexity was also observed. These observations were accompanied by increased muscle denervation and axonal damage, evinced by the increase in neural cell adhesion molecule-positive fibres (Y vs. NS: P < 0.0001; Y vs. S: P = 0.02) and the increase in serum concentration of neurofilament light chain (P < 0.0001), respectively. Notably, most of these MU and NMJ parameters did not differ when comparing older individuals with or without sarcopenia.
Conclusions: Alterations in MU properties, axonal damage, an altered innervation profile and NMJ instability are prominent features of the ageing of the neuromuscular system. These neuromuscular alterations are accompanied by muscle wasting and weakness; however, they appear to precede clinically diagnosed sarcopenia, as they are already detectable in older NS individuals.
Keywords: electromyography; fibre denervation; motoneuron; motor units; muscle atrophy; neuromuscular junction.
© 2024 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures

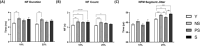


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

