Hunger signalling in the olfactory bulb primes exploration, food-seeking and peripheral metabolism
- PMID: 39236785
- PMCID: PMC11471258
- DOI: 10.1016/j.molmet.2024.102025
Hunger signalling in the olfactory bulb primes exploration, food-seeking and peripheral metabolism
Abstract
Objective: Although the metabolic state of an organism affects olfactory function, the precise mechanisms and their impact on behavior and metabolism remain unknown. Here, we assess whether ghrelin receptors (GHSRs) in the olfactory bulb (OB) increase olfactory function and influence foraging behaviors and metabolism.
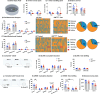
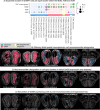
Methods: We performed a detailed behavioural and metabolic analysis in mice lacking GHSRs in the OB (OBGHSR deletion). We also analsyed OB scRNA-seq and spatial transcriptomic datasets to assess GHSR+ cells in the main and accessory olfactory bulbs, as well as the anterior olfactory nucleus.
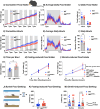
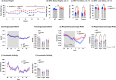
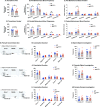
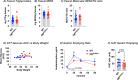
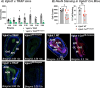
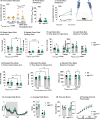
Results: OBGHSR deletion affected olfactory discrimination and habituation to both food and non-food odors. Anxiety-like and depression-like behaviors were significantly greater after OBGHSR deletion, whereas exploratory behavior was reduced, with the greatest effect under fasted conditions. OBGHSR deletion impacted feeding behavior as evidenced by altered bout number and duration, as well as buried food-seeking. OBGHSR deletion increased body weight and fat mass, spared fat utilisation on a chow diet and impaired glucose metabolism indicating metabolic dysfunction. Cross referenced analysis of OB scRNA-seq and spatial transcriptomic datasets revealed GHSR+ glutamate neurons in the main and accessory olfactory bulbs, as well as the anterior olfactory nucleus. Ablation of glutamate neurons in the OB reduced ghrelin-induced food finding and phenocopied results seen after OBGHSR deletion.
Conclusions: OBGHSRs help to maintain olfactory function, particularly during hunger, and facilitate behavioral adaptations that optimise food-seeking in anxiogenic environments, priming metabolic pathways in preparation for food consumption.
Keywords: Anxiety; Ghrelin; Glutamate; Hunger; Metabolism; Olfaction; Olfactory bulb; Transcriptomics.
Copyright © 2024 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of competing interest J.M.Z. receives research funding from Novo Nordisk for another project and consulted for Helsinn Healthcare S.A. and Dexcel Pharma Technologies Ltd. during the time these studies were performed. The other authors have nothing to disclose. All other authors report no conflict of interest.
Figures









References
-
- Jovanovic P., Riera C.E. Olfactory system and energy metabolism: a two-way street. Trends Endocrinol Metabol. 2022;33(4):281–291. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

